Thermal Stability of Ginkgolic Acids and Thermal Decomposition Kinetics of Ginkgolic Acid C15:1
-
摘要: 为研究银杏酸的热稳定性及热分解动力学,采用高效液相色谱-电喷雾电离-质谱确定银杏酸70 ℃热分解产物,以热重分析法对银杏酸C15:1的热分解行为及其动力学规律进行考察。在静止空气气氛下,控制升温速率为5.0、10.0、15.0和20.0 ℃·min−1,记录银杏酸C15:1的热重-差示扫描量热曲线,运用Ozawa-Flynn-Wall、Friedman和Šatava-Šesták、Coats-Redfern法计算银杏酸C15:1的热分解参数。结果表明银杏酸70 ℃加热30 d后有少量银杏酚产生。银杏酸C15:1热重曲线上最明显的失重发生在第一失重阶段,失重速率峰温度243 ℃,失重率为14.37%,为银杏酸C15:1的脱羧分解。计算得出银杏酸C15:1热脱羧反应表观活化能为67.44 kJ·mol−1。银杏酸C15:1脱羧动力学模型函数系Avrami-Erofeev方程,其积分形式为G(α)=[−ln(1−α)]1/2,分解机理为随机成核和随后生长,反应级数n=1/2。该研究为银杏制品中银杏酸含量的控制提供帮助。Abstract: Thermal stability of ginkgolic acids and thermal decomposition kinetics ginkgolic acid C15:1 were investigated. Ginkgolic acid was heated at 70 ℃ for 30 d, a small amount of ginkgols were determined by liquid chromatography electrospray spray ionization mass spectrometry (LC-ESI-MS). The thermal decomposition behavior and kinetics of ginkgolic acid C15:1 were investigated by thermogravimetric analysis (TG) under the static air atmosphere at the heating rates of 5.0, 10.0, 15.0 and 20.0 ℃·min−1. The thermogravimetric differential scanning curve of ginkgolic acid C15:1 was recorded and the thermal decomposition parameters were calculated by Ozawa-Flynn-Wall, Friedman, Šatava-Šesták and Coats-Redfern methods. The most obvious weight loss on the TG curves of ginkgolic acid C15:1 occurred in the first weight loss stage, the peak temperature was 243 ℃, and the weight loss rate was 14.37%, which was the decarboxylation reaction of ginkgolic acid C15:1. The calculated activation energy using Ozawa-Flynn-Wall and Friedman method was 67.44 kJ·mol−1. The dynamic model function of ginkgolic acid C15:1 decarboxylation was Avrami-Erofeev equation, its integral form was G(α)=[−ln(1−α)]1/2, and the decomposition mechanism was random nucleation and subsequent growth, and the reaction order was n=1/2. This study would provide help for the control of silver apricot acid content in ginkgo products.
-
银杏(Ginkgo biloba L.)为我国珍贵树种,其叶、果具有极高的经济价值。以银杏叶提取物为原料生产的药品或保健食品是目前世界上十大最畅销植物类产品之一[1]。银杏果实俗称白果,新鲜白果常直接烹饪为菜肴,大部分白果干燥后被加工成白果粉添加到糕点、饮料中[2]。银杏的叶和果中除了含有黄酮和内酯等活性成分外,还含有一类具有毒副作用的烷基酚酸类化合物——银杏酸,在银杏叶提取物生产和白果加工过程中被要求除去[2-4]。
银杏酸(Ginkgolic acids)属漆酚类物质,为6-烷基或烯基水杨酸衍生物的同系混合物,由银杏酸C13:0、C15:0、C15:1、C17:1、C17:2五个同系物组成,其中以银杏酸C15:1含量最高[3],银杏酸C15:1(白果新酸)也是中国药典银杏酸同系物检测中的定位对照品[5]。由于具有致敏毒性[4,6],2020年国家药典规定银杏酸在银杏叶提取物中的含量不得超过5 ppm[5]。为此,各种方法被用于控制、降低银杏制品中银杏酸的含量,如水杨酸脱羧酶降解,可使银杏酸降解率达到40%左右[7];采用乳酸菌发酵白果汁,可减少其中70%的银杏酸[8];高温热炒短时间就可明显降低白果中银杏酸含量[9];水蒸气和微波加热可以减少白果中54.5%和27%的银杏酸[10]。其中加热法可以保持白果或银杏叶的原始形态而应用更为广泛。研究银杏酸的热稳定性和热分解过程对白果和银杏叶热处理过程控制银杏酸有重要的指导意义。本项目组前期研究了银杏酸在水中的溶解性[11]及银杏酸的光热稳定性[12],发现银杏酸固体在70 ℃加热30 d,其在310 nm处的吸收有较明显降低,在250 ℃加热30 min,可使银杏酸脱羧生成银杏酚[13]。但70 ℃条件下银杏酸的降解产物是否为银杏酚并没有实验证实,且有关银杏酸单体的热分解动力学国内外尚未见报道。
热重分析(TG)是在程序控温条件下测量物质质量变化与温度关系的一类方法,能够快速准确测定固体化合物的熔融、晶型转变、分解等变化。本文拟采用高效液相色谱-电喷雾电离-质谱(LC-ESI-MS)分析银杏酸70 ℃加热30 d的降解产物,并在分离得到银杏酸C15:1单体基础上,采用非等温TG法,研究其热分解动力学,为银杏制品热加工过程中降低银杏酸含量提供理论基础。
1. 材料与方法
1.1 材料与仪器
银杏酸同系混合物(简称银杏酸) 从银杏外种皮中分离得到,由银杏酸C13:0(20.7%)、C15:0(3.3%)、C15:1(51.6%)、C17:1(21.1%)和C17:2(3.3%)组成,高效液相色谱(HPLC)纯度98%,由本实验室自制,制备方法见参考文献[13-14],于4 ℃保存;甲醇(色谱级) Tedia 公司;娃哈哈纯净水 娃哈哈集团有限公司;其它试剂皆为分析纯 国药集团化学试剂有限公司。
Surveyor LCQ液相色谱-质谱(LC-MS)联用仪配备电喷雾离子源(ESI)、Thermo-Nicolet iS50傅里叶变换红外光谱仪 美国Thermo公司;HPLC仪配有WK500 LC-500P高压泵、SPD-500紫外检测器、Welch Ultimate AQ-C18(21.2×250 mm,10 μm)制备柱、N2000色谱工作站 杭州旭昱科技有限公司;Agilgent 1260高效液相色谱仪 包括G1312C四元泵、G1322脱气、G1314B VWD、TC-C18 column(150 mm×4.6 mm,5 μm)色谱柱 美国Agilent公司;Shimp–pack VP-ODS(4.6×250 mm,5 μm)色谱柱、UV-2450紫外可见分光光度计 日本SHIMADZU公司;Netzsch STA449C综合热分析仪 德国NETZSCH公司;PH-050A电动恒温干燥箱 上海实验仪器有限公司。
1.2 实验方法
1.2.1 银杏酸的热稳定性分析
热稳定性参见文献[13],称取银杏酸50 mg于小烧杯中封口膜封口后,于70 ℃烘箱中恒温30 d取出,LC-ESI-MS分析。色谱条件:柱温35 ℃,流动相为甲醇:1%乙酸(90:10,v/v),流量1.0 mL∙min−1,二极管整列检测器检测波长为275和310 nm。质谱条件:电喷雾电离化(ESI)负离子模式,喷雾压力1.4 MPa,鞘气(N2)流量35 arb,辅助气体流量5 arb,质量扫描范围50~1000 m/z。
1.2.2 银杏酸C15:1的制备及表征
1.2.2.1 银杏酸C15:1的制备
银杏酸以四氢呋喃:甲醇(1:1,v/v)溶解,配成200 mg/mL甲醇溶液,制备条件:柱温40 ℃,流动相为甲醇-1%HAc溶液(86:14,v/v),流速24 mL∙min−1,紫外检测波长300 nm,进样1 mL,分段收集第二个峰,分析HPLC检测峰纯度(分析条件:柱温40 ℃,流动相为甲醇-3%乙酸溶液(92:8,v/v),检测波长310 nm;流速1 mL·min−1),合并单一组分馏分,浓缩至干后得银杏酸C15:1。
1.2.2.2 紫外光谱及红外光谱
银杏酸C15:1用甲醇溶解,紫外扫描范围190~400 nm,测其紫外光谱。银杏酸C15:1以二氯甲烷为溶剂溶解,用毛细管滴加两滴在压好的KBr压片上面,待完全干燥后在400~4000 nm范围内测其红外光谱。
1.2.3 银杏酸C15:1热重和差热分析
称取银杏酸C5:1 5 mg置于氧化铝坩埚,在静态空气气氛中,分别采用5.0、10.0、15.0和20.0 ℃·min−1程序升温速率(β)升温,温度范围:室温~600 ℃,α-Al2O3为参比,分别记录热重和差示扫描量热曲线。
1.2.4 银杏酸C15:1脱羧的动力学分析条件
本研究采用Ozawa-Flynn-Wall(OFW)[15-18]、Friedman[17,19]和Šatava-Šesták[15,20]、Coats-Redfern法[21-22]来分析银杏酸C5:1的第一步热分解动力学。
OFW法为积分法的一种,适用于升温时试样有质量损失而有小分子释放过程的数学处理,通常根据一组随升温速率提高而向高温推移的热重曲线计算,若反应机制不变,将得到一组平行线[23];Friedman法为微分法的一种[17,19,23],分别用于银杏酸C15:1过程表观活化能的计算。
lgβ=lg[AERG(α)]−2.315−0.4567×ERT (1) 式中:α为失重率,%;β为升温速率,K·min−1;T为绝对温度,K;A为指前因子,min−1;E为活化能,kJ·mol−1;R摩尔气体常数,8.314 J·K−1·mol−1;G(α)为积分反应机理函数。对于G(α)来说,若α为定值,则G(α)也是固定的,因此lgβ与1/T呈线性关系。以lgβ对1/T作图,由直线的斜率即可求出活化能E。
ln[(dα/dT)β]=ln[Af(α)]−E/RT (2) 式中:f(
α )为微分反应机理函数。以ln[(dα /dT)β]对1/T作图,即可得到一条直线,活化能E可以通过直线的斜率求出。因Šatava-Šesták方程适合研究固相非定温热分解动力学[22],故采用Šatava-Šesták方程和Coats-Redfern方程进行银杏酸C15:1热分解反应动力学模型的推断。
lgG(a)=lgAERβ−2.315−0.4567×ERT (3) 式中:因为β是固定的,lg(AE/Rβ)即是一个常数,以lgG(
α )对1/T作图,通过曲线的斜率和截距即可求得Es和A。lnG(α)T2=lnARβE−ERT (4) 式中:ln(AR/βE)即是一个常数,以ln[G(
α )/T2]对1/T作图,通过曲线的斜率和截距即可求得E和A。2. 结果与分析
2.1 银杏酸的热稳定性
将银杏酸70 ℃加热30 d后的LC-ESI-MS分析结果见图1。图1A~图1B最上一张图为总离子流图(TIC),其余皆为选择离子流图。由银杏酸结构式[3],其五个同系物分子量M分别为C13:0(320)、C15:1(346)、C17:2(372)、C15:0(348)和C17:1(374);而银杏酚分子量比银杏酸少44。为了证实银杏酸在70 ℃加热后发生了脱羧反应,采用系统提供的选择离子检测功能,分别选择银杏酸各单体分子离子峰[M-H]-的质荷比(m/z)及[M-44-H]-的m/z查找,银杏酸的五个同系物及其脱羧产物的分子离子峰均找到,见图1上所标注。推测银杏酸在70 ℃加热30 d后310 nm处紫外吸收降低是部分银杏酸受热脱羧生成银杏酚所致[13]。为深入研究银杏酸热分解过程,分离出银杏酸含量最高的C15:1单体进行热重分析。
2.2 银杏酸C15:1的制备
银杏酸的HPLC制备谱图见图2,银杏酸分析常在λ=310 nm下进行,考虑到制备色谱上样量大,为兼顾上样量和灵敏度,制备色谱波长选择300 nm,流速24 mL·min−1时各组分分离效果较好,所有组分在100 min内全部出完。图2中第二个峰为银杏酸C15:1,分段收集第二个峰馏分,HPLC分析色谱检测峰的单一性,合并单一银杏酸C15:1馏分,制备过程重复两次,得到银杏酸C15:1,为浅黄色固体,其HPLC分析谱图见图3,HPLC纯度>98%。
对所得银杏酸C15:1单体进行紫外和红外扫描,分别得到其紫外和红外吸收光谱(图4)。
图4A显示出银杏酸典型的紫外吸收光谱,在310 nm处有最大吸收,与文献报道一致[13,24]。图4B的红外光谱数据与文献[24-25]一致,银杏酸C15:1的酚羟基νO-H=3010、1305、710 cm−1分别为C-O键(苯环上的C与酚羟基上的O)的νC-O伸缩振动和δO-H面外弯曲振动。银杏酸C15:1中羧基的O-H面内弯曲振动δO-H和面外弯曲振动δO-H和νC=O分别出现在1211、900和1647 cm−1[24],而3005、1634、831 cm−1分别为双键的ν=C-H、νC=C和δ=C-H,显示双键存在[22]。证实分离到的为银杏酸C15:1单体。
2.3 银杏酸C15:1的热重分析
银杏酸C15:1在不同升温速率下所得的热重(TG)曲线见图5。由图5可知,随着升温速率的增加,TG曲线逐渐向高温方向移动,即热分解阶段的温度范围发生了微小的变化,但是升温速率的大小对样品的失重率基本没有影响。这是由于快速升温导致了热滞后现象,升温速率越快,试样的起始分解温度和终止分解温度也会随升温速率的增大而提高,从而使反应曲线向高温方向移动[23]。
2.4 银杏酸C15:1的热分解过程
图6为升温速率为10 ℃/min时银杏酸C15:1的热重-差示扫描量热-微商热重(TG-DSC-DTG)曲线。由图6可见,DSC曲线的第一个吸热峰峰值为45.9 ℃,TG无相应失重,为银杏酸C15:1固体熔融峰,与银杏酸C15:1的熔点40~41 ℃接近[11]。银杏酸C15:1的TG曲线存在三个显著的失重台阶,第一步失重的起始温度为86.5 ℃,这一阶段的银杏酸单体脱去了一个羧基分解成银杏酚C15:1,失重率为14.37%(理论值为13.0%),DSC曲线显示一小吸热峰,DTG曲线上显示最大失重速率,峰值243.0 ℃。银杏酸热稳定实验的LC-ESI-MS分析显示,银杏酸70 ℃加热30 d可使银杏酸脱羧转化为银杏酚,使其在310 nm的紫外吸收略有减少[13]。受快速升温热滞后效应影响,图6中的银杏酸C15:1固体的熔融温度和脱羧起始温度都较其熔点和热稳定性实验的分解温度为高。王世霞等[26]研究苯甲酸脱羧反应时同样发现这种现象,虽然苯甲酸脱羧反应在170 ℃才能完成,但当升温到100 ℃时,其羧基拉曼谱峰已发生变化,显示此时已经发生脱羧反应。本实验结果证实银杏酸的脱羧反应在其热重分析记录的失重起始温度前已经发生,因为反应速率很小,容易被忽略。第二步失重的对应着第三个明显的吸热峰,吸热峰的峰值为273.0 ℃,这一阶段银杏酸C15:1完成脱羧反应,生成的银杏酚C15:1有可能烷基侧链断裂,形成部分聚合体,DTG曲线呈现一小的台阶。温度继续升高发生第三步明显失重,DTG曲线在430~500 ℃之间呈现明显的失重速率变化,DSC曲线显示一宽大的放热峰,为完全氧化反应。
银杏酸的脱羧产物银杏酚亦为银杏中的活性成分,具有抗肿瘤活性[3]。Lepoittevn等[6]采用豚鼠的迟发型超敏反应试验发现银杏中的银杏酸为强致敏源,而银杏酚不引起过敏反应。因此,在制备过程中通过第一步热分解将银杏酸转化为银杏酚,即可实现银杏制品中银杏酸毒性的脱除。由此,本文着重讨论银杏酸C15:1的第一级热分解反应过程。
2.5 银杏酸C15:1热分级机理分析
2.5.1 银杏酸C15:1第一级热分解活化能E求取
依据OFW法,在不同的转化率α下以lnβ对1/T作图,再用最小二乘法对曲线进行线性拟合[27-28],活化能E即可通过曲线的斜率求得见图7,计算结果列于表1中。通过OFW法计算出的银杏酸C15:1热降解第一阶段的平均活化能E为67.50 kJ·mol−1。
表 1 OFW法和Friedman法计算银杏酸C15:1热降解过程的活化能Table 1. Activation energies of degradation process of ginkgolic acid C15:1 with OFW and Friedman methods转化率α OFW法 Friedman法 E(kJ·mol−1) R2 E(kJ·mol−1) R2 0.2 67.3468 0.9980 60.8444 0.9867 0.3 66.8906 0.9986 61.2942 0.9960 0.4 66.8163 0.9993 63.7757 0.9981 0.5 67.2172 0.9997 67.1267 0.9977 0.6 67.8739 0.9999 70.8492 0.9994 0.7 68.8433 0.9999 73.4529 0.9993 0.8 69.7450 0.9999 74.2906 0.9956 平均值 67.498 67.376 对于活化能的计算,除了利用OFW法计算之外,还可通过Friedman法推算,以ln[(dα/dT)β]对1/T作图,活化能E即可通过曲线的斜率求得。Friedman法计算的银杏酸C15:1热降解第一阶段的活化能曲线图以及结果见图8和表1。由表1可知,通过Friedman法计算出的银杏酸C15:1热降解第一阶段的平均活化能为67.38 kJ·mol−1,与OFW的计算结果相近。从而得出银杏酸C15:1分解为银杏酚C15:1分解反应的平均活化能
¯E =67.44 kJ·mol−1。2.5.2 银杏酸C15:1热分解最可机理函数的推断
将文献[22]中常见的十几种机理模型函数分别带入Šatava-Šesták方程和Coats-Redfern方程中[29-30],求出不同机理函数所对应的动力学参数,仅以升温速率为10 ℃/min为例,计算结果列于表2中。将这些模型函数计算出的Es值与OFW法和Friedman法计算出的活化能的平均值
¯E 相比较,找出满足|(¯E −Es)/¯E |≤0.1的Es且相关系数大于0.99的机理函数。结果发现,只有当机理函数为G(α)=[−ln(1−α)]1/2时符合要求,Šatava-Šesták法计算出的Es=72.56 kJ·mol−1,lgA=7.74 s−1,而Coats-Redfern法计算出的Es=68.50 kJ mol−1,lgA=7.06 s−1,其结果与OFW法,Friedman法的E值较接近,相关系数数值较大。因此可以判断,银杏酸C15:1热分解第一阶段最可能机理函数,即Avrami-Erofeev方程,其非等温热降解机理为随机成核和随后生长,反应级数n=1/2,其积分形式为G(α)=[−ln(1−α)]1/2,微分形式为f(α)=2(1−α)[−ln(1−α)]1/2。表 2 银杏酸C15:1热降解过程的动力学参数Table 2. Kinetic parameters of the thermal degradation of ginkgolic acid C15:1G(α) Satava-Sestak法 Coats-Redfern法 Es
(kJ·mol−1)lgA(s−1) R2 Es
(kJ·mol−1)lgA(s−1) R2 α1/3 34.3466 3.5915 0.9963 28.3196 2.2128 0.9937 α1/2 51.5204 5.3617 0.9963 46.3794 4.3734 0.9948 α 103.0408 10.8997 0.9963 100.5578 10.5485 0.9957 α2 206.0817 22.2766 0.9963 208.9146 22.5439 0.9960 (1−2α/3)−(1-α)2/3 239.5822 25.2954 0.9997 244.1434 25.6959 0.9997 [−ln(1−α)]1/2 72.5590 7.7453 0.9967 68.50337 7.0751 0.9961 [−ln(1−α)]1/3 48.3721 5.1310 0.9967 43.0687 4.0831 0.9956 [−ln(1−α)]1/4 36.2795 3.8608 0.9967 30.3522 2.5359 0.9951 1−(1−α)1/2 122.4257 12.8595 0.9998 120.9427 12.6634 0.9998 1−(1−α)1/3 129.6143 13.5237 0.9995 128.5022 13.3786 0.9994 −ln(1−α) 145.1179 15.8156 0.9967 144.8057 15.7715 0.9964 1/(1−α) 98.7616 11.1068 0.8772 94.7941 10.5676 0.8537 (1−α)−1−1 200.6059 22.3336 0.9765 203.1562 22.5771 0.9748 3. 结论
根据热分析,银杏酸C15:1在空气中的热分解为银杏酸脱羧转化为银杏酚主分解阶段和侧链不饱和银杏酚缩合,及银杏酸的完全氧化三个阶段,其中第一步热分解使银杏酸脱羧形成银杏酚,消除了银杏酸的毒性。分别用Ozawa-Flynn-Wall法和Friedman法计算出银杏酸C15:1热分解第一阶段的活化能分别为67.4981、67.3762 kJ·mol-1。另外,根据Šatava-Šesták方程和Coats-Redfern方程推断出银杏酸C15:1热降解反应的动力学模型函数系Avrami-Erofeev方程,其积分形式为G(α)=[−ln(1−α)]1/2,微分形式为f(α)=2(1−α)[−ln(1−α)]1/2,降解机理为随机成核和随后生长,反应级数n=1/2。
-
表 1 OFW法和Friedman法计算银杏酸C15:1热降解过程的活化能
Table 1 Activation energies of degradation process of ginkgolic acid C15:1 with OFW and Friedman methods
转化率α OFW法 Friedman法 E(kJ·mol−1) R2 E(kJ·mol−1) R2 0.2 67.3468 0.9980 60.8444 0.9867 0.3 66.8906 0.9986 61.2942 0.9960 0.4 66.8163 0.9993 63.7757 0.9981 0.5 67.2172 0.9997 67.1267 0.9977 0.6 67.8739 0.9999 70.8492 0.9994 0.7 68.8433 0.9999 73.4529 0.9993 0.8 69.7450 0.9999 74.2906 0.9956 平均值 67.498 67.376 表 2 银杏酸C15:1热降解过程的动力学参数
Table 2 Kinetic parameters of the thermal degradation of ginkgolic acid C15:1
G(α) Satava-Sestak法 Coats-Redfern法 Es
(kJ·mol−1)lgA(s−1) R2 Es
(kJ·mol−1)lgA(s−1) R2 α1/3 34.3466 3.5915 0.9963 28.3196 2.2128 0.9937 α1/2 51.5204 5.3617 0.9963 46.3794 4.3734 0.9948 α 103.0408 10.8997 0.9963 100.5578 10.5485 0.9957 α2 206.0817 22.2766 0.9963 208.9146 22.5439 0.9960 (1−2α/3)−(1-α)2/3 239.5822 25.2954 0.9997 244.1434 25.6959 0.9997 [−ln(1−α)]1/2 72.5590 7.7453 0.9967 68.50337 7.0751 0.9961 [−ln(1−α)]1/3 48.3721 5.1310 0.9967 43.0687 4.0831 0.9956 [−ln(1−α)]1/4 36.2795 3.8608 0.9967 30.3522 2.5359 0.9951 1−(1−α)1/2 122.4257 12.8595 0.9998 120.9427 12.6634 0.9998 1−(1−α)1/3 129.6143 13.5237 0.9995 128.5022 13.3786 0.9994 −ln(1−α) 145.1179 15.8156 0.9967 144.8057 15.7715 0.9964 1/(1−α) 98.7616 11.1068 0.8772 94.7941 10.5676 0.8537 (1−α)−1−1 200.6059 22.3336 0.9765 203.1562 22.5771 0.9748 -
[1] 宋洋, 于志斌, 尤晓敏, 等. 我国银杏叶提取物市场发展现状、挑战与对策[J]. 中国新药杂志,2015,24(23):2651−2655. [SONG Y, YU Z B, YOU X M, et al. The development status, challenges and countermeasures of Ginkgo biloba extract market in China[J]. Chinese Journal of New Drugs,2015,24(23):2651−2655. SONG Y, YU Z B, YOU X M, et al. The development status, challenges and countermeasures of Ginkgo biloba extract market in China[J]. Chinese Journal of New Drugs, 2015, 24(23): 2651-2655.
[2] 张风景, 顾正彪, 李兆丰, 等. 不同干燥方式对银杏全粉品质的影响[J]. 食品工业科技,2017,38(9):196−205. [ZHANG F J, GU Z B, LI Z F, et al. Effects of different drying methods on the quality of ginkgo powder[J]. Science and Technology of Food Industry,2017,38(9):196−205. ZHANG F J, GU Z B, LI Z F, et al. Effects of different drying methods on the quality of ginkgo powder[J]. Science and Technology of Food Industry, 2017, 38(9): 196-205.
[3] YANG X M, ZHONG X L, CHEN Y C, et al. LC method for determination of ginkgolic acids in mice plasma and its application to a pharmacokinetic study[J]. Chromatographia,2009,69(5/6):593−596.
[4] 房仙颖, 谢莹莹, 章祎唯, 等. 银杏酸毒副作用及朴树提取物对其减毒效果研究[J]. 林产化学与工业,2020,40(6):99−106. [FANG X Y, XIE Y Y, ZHANG Y W, et al. Toxic and side effects of ginkgolic acid and effect of Celtis sinensis extracts on detoxification of ginkgolic acid[J]. Chemistry and Industry of Forest Products,2020,40(6):99−106. doi: 10.3969/j.issn.0253-2417.2020.06.013 FANG X Y, XIE Y Y, ZHANG Y W, et al. Toxic and side effects of ginkgolic acid and effect of Celtis sinensis extracts on detoxification of ginkgolic acid[J]. Chemistry and Industry of Forest Products, 2020, 40(6): 99-106. doi: 10.3969/j.issn.0253-2417.2020.06.013
[5] 国家药典委员会. 中华人民共和国药典, 2020版第一部, 植物油脂和提取物, 银杏叶提取物[M]. 北京: 中国医药科技出版社, 2020 National Pharmacopoeia Committee. Pharmacopoeia of the people's Republic of China, Part 1, 2020 edition, vegetable oils and extracts, Ginkgo biloba extract[M]. Beijing: China Pharmaceutical Science and Technology Press, 2020.
[6] LEPOITTEVIN J P, BENEZRA C, ASAKAWA Y. Allergic contact dermatitia to Ginkgo biloba L. : Relationship with urushiol[J]. Archives of Dermatological Research,1989,281:227−230. doi: 10.1007/BF00431055
[7] HU Y, HUA Q, SUN G, et al. The catalytic activity for ginkgolic acid biodegradation, homology modeling and molecular dynamic simulation of salicylic acid decarboxylase[J]. Computational Biology and Chemistry,2018,75:82−90. doi: 10.1016/j.compbiolchem.2018.05.003
[8] WANG Y, TAO Y, ZHANG X, et al. Metabolic profile of ginkgo kernel juice fermented with lactic aicd bacteria: A potential way to degrade ginkgolic acids and enrich terpene lactones and phenolics[J]. Process Biochemistry,2019,75:25−33.
[9] 李转梅, 张学兰, 李慧芬, 等. 白果不同部位及不同炮制品种白果酸和总银杏酸定量比较[J]. 中成药,2015,37(1):164−168. [LI Z M, ZHANG X L, LI H F, et al. Quantitative comparison of ginkgolic acid and total ginkgolic acids in different parts and processed products of Ginkgo semen[J]. Chinese Traditional Patent Medicine,2015,37(1):164−168. doi: 10.3969/j.issn.1001-1528.2015.01.035 LI Z M, ZHANG X L, LI H F, et al. Quantitative comparison of ginkgolic acid and total ginkgolic acids in different parts and processed products of Ginkgo semen[J]. Chinese Traditional Patent Medicine, 2015, 37(1): 164-168. doi: 10.3969/j.issn.1001-1528.2015.01.035
[10] FAN G J, WANG X, WU C E, et al. Effect of heating on the content and composition of ginkgolic acids in ginkgo seeds[J]. Quality Assurance and Safety of Crops & Foods,2017,9(2):195−199.
[11] 杨小明, 方洋洋, 李月英, 等. 银杏酸C15: 1在水中溶解度的测定及关联[J]. 江苏大学学报(自然科学版),2015,36(5):557−560. [YANG X M, FANG Y Y, LI Y Y, et al. Measurement and correlation of solubility for ginkgolic acid C15: 1 in water[J]. Journal of Jiangsu University (Natural Science Edition),2015,36(5):557−560. YANG X M, FANG Y Y, LI Y Y, et al. Measurement and correlation of solubility for ginkgolic acid C15: 1 in water[J]. Journal of Jiangsu University (Natural Science Edition), 2015, 36(5): 557-560.
[12] 杨小明, 何军, 柳艳君, 等. 银杏酸的荧光特征和光热稳定性研究[J]. 时珍国医国药,2009,20(10):2453−2454. [YANG X M, HE J, LIU Y J, et al. The flurescence characterristics of ginkgolic acid and heat and sunlight stability[J]. Lishizhen Medicine and Materia Medica Research,2009,20(10):2453−2454. doi: 10.3969/j.issn.1008-0805.2009.10.036 YANG X M, HE J, LIU Y J, et al. The flurescence characterristics of ginkgolic acid and heat and sunlight stability[J]. Lishizhen Medicine and Materia Medica Research, 2009, 20(10): 2453-2454. doi: 10.3969/j.issn.1008-0805.2009.10.036
[13] YANG X M, WANG Y F, LI Y Y, et al. Thermal stability of ginkgolic acids from Ginkgo biloba and the effects of ginkgol C17: 1 on the apoptosis and migration of SMMC7721 cells[J]. Fitoterapia,2014,98:66−76. doi: 10.1016/j.fitote.2014.07.003
[14] 杨小明, 陈盛霞, 张荣仙, 等. 银杏外种皮石油醚提取物的杀钉螺活性研究[J]. 中国人兽共患病学报,2006,22(10):961−964. [CHEN S X, YANG X M, ZHANG L X, et al. Study on molluscicidal effect of the extracts from sarcotesta of Ginkgo biloba[J]. Chinese Journal of Parasitology and Parasitic Diseases,2006,22(10):961−964. doi: 10.3969/j.issn.1002-2694.2006.10.017 CHEN S X, YANG X M, ZHANG L X, et al. Study on molluscicidal effect of the extracts from sarcotesta of Ginkgo biloba[J]. Chinese Journal of Parasitology and Parasitic Diseases, 2006, 22(10): 961-964. doi: 10.3969/j.issn.1002-2694.2006.10.017
[15] 牛芊, 周彩荣, 詹自力. 甲硫氨酸的非等温热分解动力学的研究[J]. 高校化学工程学报,2018,32(5):1112−1118. [NIU Q, ZHOU C R, ZHAN Z L. Study on non-isothermal decomposition kinetics of methionine[J]. Journal of Chemical Engineering of Chinese Universities,2018,32(5):1112−1118. doi: 10.3969/j.issn.1003-9015.2018.05.016 NIU Q, ZHOU C R, ZHAN Z L. Study on non-isothermal decomposition kinetics of methionine[J]. Journal of Chemical Engineering of Chinese Universities, 2018, 32(5): 1112-1118. doi: 10.3969/j.issn.1003-9015.2018.05.016
[16] 祝远娇, 陈小鹏, 王琳琳, 等. 脱氢枞酸在空气中的热分解动力学[J]. 化工学报,2008,59(10):2526−2529. [ZHU Y J, CHEN X P, WANG L L, et al. Thermal decomposition kinetics of dehydroabietic acid in air[J]. Journal of Chemical Industry and Engineering (China),2008,59(10):2526−2529. doi: 10.3321/j.issn:0438-1157.2008.10.016 ZHU Y J, CHEN X P, WANG L L, et al. Thermal decomposition kinetics of dehydroabietic acid in air[J]. Journal of Chemical Industry and Engineering (China), 2008, 59(10): 2526-2529. doi: 10.3321/j.issn:0438-1157.2008.10.016
[17] TITA M E B, TITA I C, VICAS T J. Thermal behaviour and kinetic study of amygdalin[J]. Journal of Thermal Analysis and Calorimetry,2018,134:765−772. doi: 10.1007/s10973-018-7263-2
[18] 杨鹏, 王展, 沈汪洋, 等. 6-姜酚/麦芽糖基-β-环糊精包合物的热分解机理研究[J]. 食品工业科技,2018,39(22):46−49. [YANG P, WANG Z, SHEN W Y, et al. Thermal decomposition mechanism study of 6-gingerol/maltosyl-β-cyclodextrin inclusion complex[J]. Science and Technology of Food Industry,2018,39(22):46−49. YANG P, WANG Z, SHEN W Y, et al. Thermal decomposition mechanism study of 6-gingerol/maltosyl-β-cyclodextrin inclusion complex[J]. Science and Technology of Food Industry, 2018, 39(22): 46-49.
[19] 王韶旭, 谭志诚, 车如心, 等. 丹酚酸B的热稳定性及其热分解动力学研究[J]. 化学学报,2012,70(2):212−216. [WANG S X, TAN Z C, CHE R X, et al. Thermal stability and kinetics of thermal decomposition of salvianolic acid B[J]. Acta Chimica Sinica,2012,70(2):212−216. doi: 10.6023/A1104075 WANG S X, TAN Z C, CHE R X, et al. Thermal stability and kinetics of thermal decomposition of salvianolic acid B[J]. Acta Chimica Sinica, 2012, 70(2): 212-216. doi: 10.6023/A1104075
[20] LIU Y, YANG L, MA C, et al. Thermal behavior of sweet potato starch by non-isothermal thermogravimetric analysis[J]. Materials,2019,12:699. doi: 10.3390/ma12050699
[21] RASOOL T, NAJAR I, SRIVASTAVA V C, et al. Pyrolysis of almond (Prunus amygdalus) shells: Kinetic analysis, modelling, energy assessment and technical feasibility studies[J]. Bioresource Technology,2021,337:125446. doi: 10.1016/j.biortech.2021.125446
[22] 胡荣祖, 史启祯. 热分析动力学[M]. 北京: 科学出版社, 2008 HU R Z, SI Q Z. Thermal analysis kinetics[M]. Beijing: Science Press, 2008.
[23] 刘振海, 张洪林. 分析化学手册⑧热分析与量热学(第三版)[M]. 北京: 化学工业出版社, 2016 LIU Z H, ZHANG H L. Handbook of analytical chemistry, No 8, thermal analysis and calorimetry (third edition)[M]. Beijing: Chemical Industry Press, 2018.
[24] 马景哲. 银杏酸单体的纯化制备及分析方法的研究[D]. 北京: 北京化工大学, 2011 MA J Z. Purification, preparation and analysis of ginkgolic acid monomer[D]. Beijing: Beijing University of Chemical Technology, 2008.
[25] 谭卫红, 沈兆邦, 王成章, 等. 银杏叶中烷基酚化合物的分离与鉴定[J]. 林产化学与工业,2001,21(4):1−6. [TAN W H, SHEN Z B, WANG C Z, et al. Isolation and identification of alkylphenols from Ginkgo biloba leaves[J]. Chemistry and Industry of Forest Products,2001,21(4):1−6. doi: 10.3321/j.issn:0253-2417.2001.04.001 TAN W H, SHEN Z B, WANG C Z, et al. Isolation and identification of alkylphenols from Ginkgo biloba leaves[J]. Chemistry and Industry of Forest Products, 2001, 21(4): 1-6. doi: 10.3321/j.issn:0253-2417.2001.04.001
[26] 王世霞, 郑海飞. 苯甲酸脱羧反应过程的拉曼光谱研究[J]. 光谱与光谱分析,2009,29(12):3312−3314. [WANG S X, ZHENG H F. Research on raman spectra of benzoic acid during decarboxylic process[J]. Spectroscopy and Spectral Analysis,2009,29(12):3312−3314. WANG S X, ZHENG H F. Research on raman spectra of benzoic acid during decarboxylic process[J]. Spectroscopy and Spectral Analysis, 2009, 29(12): 3312- 3314.
[27] 何翊, 孙挺. 1-甲基环丙烯与β-环糊精的相互作用及热分解动力学[J]. 食品科学,2006,27(8):25−28. [HE Y, SUN T. Interaction and thermal decomposition kinetics between 1-methylcyclopropene and β-cyclodextrin[J]. Food Science,2006,27(8):25−28. doi: 10.3321/j.issn:1002-6630.2006.08.001 HE Y, SUN T. Interaction and thermal decomposition kinetics between 1-methylcyclopropene and β-cyclodextrin[J]. Food Science, 2006, 27(8): 25-28. doi: 10.3321/j.issn:1002-6630.2006.08.001
[28] 刘建群, 高俊博, 刘小红. 热分析法研究冬凌草甲素的热稳定性和分解动力学[J]. 林产化学于工业,2015,35(6):119−125. [LIU J Q, GAO J B, LIU X H. Thermal stability and decomposition kinetics of oridonin by thermal analysis method[J]. Chemistry and Industry of Forest Products,2015,35(6):119−125. LIU J Q, GAO J B, LIU X H. Thermal stability and decomposition kinetics of oridonin by thermal analysis method[J]. Chemistry and Industry of Forest Products, 2015, 35(6): 119-125.
[29] 王韶旭, 赵波, 谭志诚, 等. 丙硫异烟胺的热稳定性及其热分解动力学[J]. 物理化学学报,2007,23(9):1459−1462. [WANG Z X, ZHAO Z, TAN Z C, et al. Thermal stability and kinetics of thermal decomposition for protionamide[J]. Acta Phys-Chin Sin,2007,23(9):1459−1462. doi: 10.3866/PKU.WHXB20070929 WANG Z X, ZHAO Z, TAN Z C, et al. Thermal stability and kinetics of thermal decomposition for protionamide[J]. Acta Phys-Chin Sin, 2007, 23(9): 1459-1462. doi: 10.3866/PKU.WHXB20070929
[30] 崔波, 金征宇, 周万里, 等. 葡萄糖基(α-1→6)-β-环糊精的热分解动力学研究[J]. 食品工业科技,2006,27(12):55−57. [CUI B, JIN Z Y, ZHOU W L, et al. Study on thermal decomposition kinetics of glucose group (α-1→6)-β-cyclodextrin[J]. Science and Technology of Food Industry,2006,27(12):55−57. doi: 10.3969/j.issn.1002-0306.2006.12.015 CUI B, JIN Z Y, ZHOU W L, et al. Study on thermal decomposition kinetics of glucose group (α-1→6)-β-cyclodextrin[J]. Science and Technology of Food Industry, 2006, 27(12): 55-57. doi: 10.3969/j.issn.1002-0306.2006.12.015





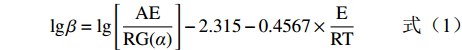


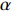
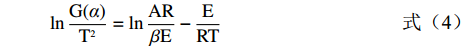

 下载:
下载:











 下载:
下载:
