Research Progress on the Effects and Mechanisms of L-carnitine on Improving Metabolic Syndrome
-
摘要: 代谢综合征是一组以肥胖、血脂异常、高血压、高尿酸血症、高血糖以及胰岛素抵抗等为主要特征的临床综合征,是心血管疾病和糖尿病的重要危险因素。研究表明,左旋肉碱,即L-肉碱,对代谢综合征具有潜在改善作用。本文对人体L-肉碱的来源及L-肉碱改善代谢综合征主要组分,如肥胖、血脂异常等的作用及机制进行综述,并讨论了L-肉碱作为氧化三甲胺前体物质的潜在健康风险问题,为代谢综合征的防治及L-肉碱的合理摄食补充及应用提供理论依据及参考。Abstract: Metabolic syndrome is a group of clinical syndromes, including obesity, dyslipidemia, hypertension, hyperuricemia, hyperglycemia and insulin resistance. These syndromes are critical risk factors for cardiovascular disease and diabetes. Recent studies indicate that L-carnitine exhibits potential improvement effects on metabolic syndrome. In the current study, the sources of L-carnitine for human and the effects and mechanisms of L-carnitine on the major components of metabolic syndrome, such as obesity and dyslipidemia, etc were summarized. The potential health risk of L-carnitine by serving as a dietary precursor for trimethylamine N-oxide was also discussed. This study provides theoretical basis and references for the treatment of metabolic syndrome and the rational dietary supplement and utilization of L-carnitine.
-
Keywords:
- L-carnitine /
- metabolic syndrome /
- improvement effects /
- mechanisms /
- trimethylamine N-oxide
-
代谢综合征(metabolic syndrome,MS)是一组由遗传因素和环境因素共同决定的症候群,包括肥胖、血脂异常、高血糖、胰岛素抵抗(IR)、高血压、高尿酸血症等[1]。MS是糖尿病、心血管疾病等慢性病的前期病理基础及危险因素。据国际糖尿病联盟估计,全球患有MS的人口约25%,其中成年人约占21.9%[2]。据统计,我国MS患病率已达20%~35%,并且仍在逐年上升[3]。MS患病率增加导致死亡率、致残率和医疗费用的持续增加,已对公众健康造成重大威胁[1]。寻求有效的营养干预措施,对MS的防治及维护人类健康具有重要意义。研究提示,摄食补充左旋肉碱或可有效改善MS相关的代谢异常指标[4]。因此,本文对人体左旋肉碱的来源以及其改善MS主要组分的作用及机制做以下综述。
1. 左旋肉碱的性质及来源
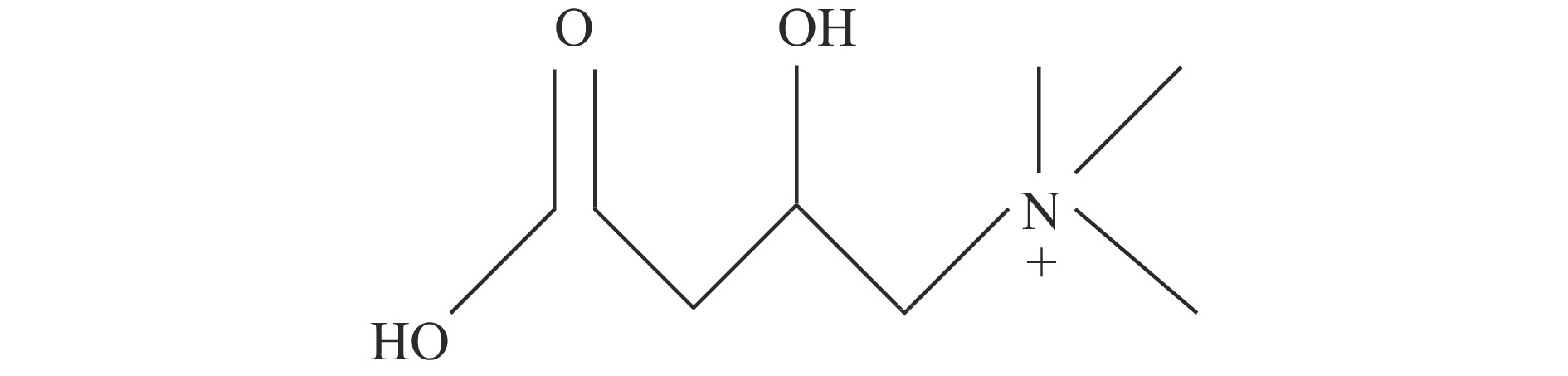
左旋肉碱(L-carnitine)别称L-肉碱,是一种类氨基酸物质,属维生素B族,化学名为L-β-羟-γ-三甲铵丁酸,分子式C7H15NO3。在L型、D型和DL型3种光学旋光体中,只有L-肉碱具有生理活性,被广泛应用于食品、医药等领域,通常被简称为“肉碱”。而D-肉碱和DL-肉碱则会竞争性的抑制肉碱乙酰转移酶(CAT)和肉碱脂肪酰转移酶(PTC)的活性,阻碍细胞的正常脂代谢[5]。在生理pH条件下,L-肉碱为季铵盐阳离子复合物,呈兼性离子,它含有1个带正电的季铵基和1个带负电的羧基,中间由1条带羟基的三碳短链相连,该羟基能与脂肪酸通过酯键结合(图1)。
L-肉碱广泛存在于自然界动、植物及微生物体中。人体含有大约300 mg/kg L-肉碱,大部分以游离形式存在,少部分以酰基肉碱形式存在[6],其中98%在细胞内,80%存在于肌肉,5%~10%存在于胃肠道,3%存在于肝脏,血液约占0.25%[7]。据估计,健康成人血清L-肉碱浓度约25~50 μmol/L,血清里游离L-肉碱含量小于20 μmol/L被定义为肉碱缺乏症。人体内L-肉碱来源主要包括内源合成和外源摄取两种方式,其中约75%来自外源食物摄取。在均衡的饮食中,人类需每天摄入约10~100 mg L-肉碱,其中大部分来自动物性食物,其中含量最高的是袋鼠(6370 mg/kg干重(dry matter,DM))和马(4230 mg/kg DM),牛排(2320 mg/kg DM)次之,猪肌肉中L-肉碱含量约830 mg/kg DM,鸡肌肉中L -肉碱含量约732 mg/kg DM[8]。海产品中L-肉碱含量约100~500 mg/kg DM[9],蔬菜、水果等植物性食品中L-肉碱含量最少,约0~50 mg/kg DM[10]。
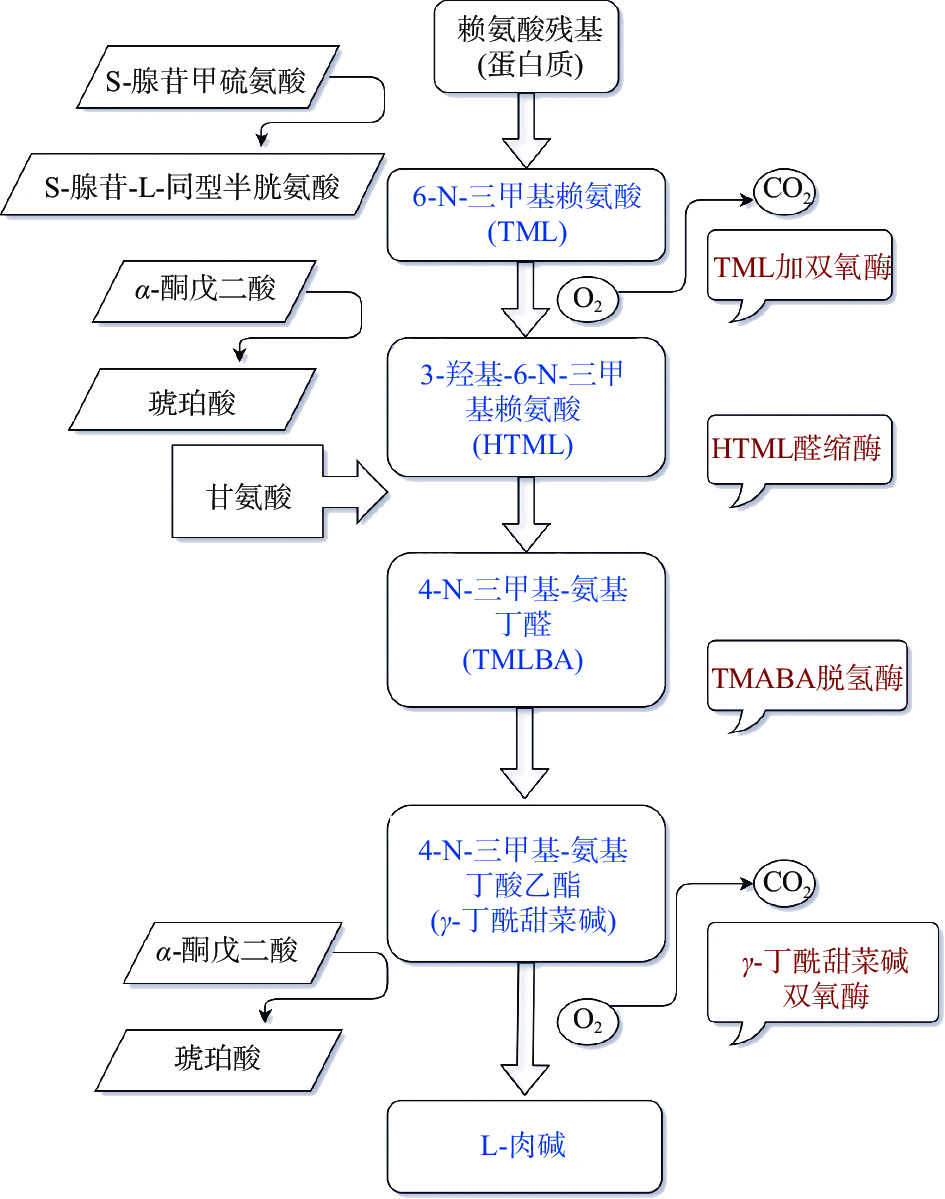
除外源性食物摄入以外,大约有25%的L-肉碱在体内合成。L-肉碱的合成主要发生在肝脏,在肾、附睾和大脑等器官中也能合成一部分。合成L-肉碱的前体是甲硫氨酸和赖氨酸,赖氨酸提供碳骨架,而甲硫氨酸提供甲基。首先由特定的甲基转移酶(赖氨酸甲基转移酶)催化富含赖氨酸的蛋白质,再经由S-腺苷甲硫氨酸为甲基供体进行N-甲基化,将多肽链中的赖氨酸转化为6-N-三甲基赖氨酸残基(TML),随后通过溶酶体内蛋白酶水解作用生成6-N-三甲基赖氨酸,然后通过线粒体内双加氧酶TML(TMLD)羟基化生成3-羟三甲基赖氨酸(HTML)。接着是HTML醛缩酶(HTMLA)催化裂解成甘氨酸和4-三甲氨基丁基醛(TMABA),TMABA脱氢酶(TMABA DH)催化脱氢生成γ-丁酰甜菜碱(GBB)。最后由γ-丁酰甜菜碱双加氧酶(BBD)驱动,在铁离子和抗坏血酸辅助因子的作用下,丁甜菜碱3位羟基化生成L-肉碱[10](图2)。
目前,人类对外源L-肉碱的每日需求量尚不明确,对儿童和成人均尚无相应的推荐摄入量(RNI)、适宜摄入量(AI)和可耐受最高摄入量(UL)等膳食建议。一般来说,成人能够自己合成维持正常代谢所需的L-肉碱浓度,婴儿L-肉碱合成能力仅为成人的15%,但母乳或牛乳奶粉喂养可维持婴儿正常L-肉碱需求。研究表明,作为营养补充剂,L-肉碱的安全性较高。雌性大鼠经口灌胃L-肉碱的半数致死剂量(LD50)为6.9 mg/kg,雄性大鼠LD50为4.9 mg/kg[11]。有报道称成人摄食补充超过3 g/d剂量的L-肉碱才会引起轻度的不良反应[12],包括恶心、呕吐、腹部绞痛、腹泻等[13]。
2. 左旋肉碱与肥胖
肥胖是一种以在脂肪组织中脂肪过量沉积为特征的慢性疾病,一般认为中心性肥胖是MS的重要始发因素。Fritz等[14]于1958年首次发现L-肉碱对肌肉和心脏组织中的长链脂肪酸的氧化具有重要作用,可以提高脂肪的β-氧化速率,加速细胞(线粒体)对脂肪的消耗。
目前有大量动物实验结果表明L-肉碱有益于治疗肥胖。Esmail等[15]在高脂饲养的成年雄性Wister大鼠中发现,250 mg/kg的L-肉碱连续口服灌胃6周可降低大鼠的血脂水平,并明显降低大鼠体重。Trkay等[16]通过每周3次在开始运动前给予肥胖大鼠1 g/kg L-肉碱干预发现,12周后肥胖大鼠的体重明显减轻。Mun等[17]发现,高脂饮食小鼠饮食添加0.5%的L-肉碱12周后,其体重、白色脂肪组织的重量及血清瘦素的浓度均显著低于模型组。实验室前期研究发现,摄食补充0.5% L-肉碱12周,可显著预防高脂饮食喂食的雄性及雌性小鼠体重及白色脂肪的增加[18]。人群研究证据同样表明L-肉碱具有良好的减肥功效。2020年,一项基于43篇随机临床对照实验(RCT)的meta分析结果显示,摄食补充L-肉碱对肥胖及非肥胖人群体重、BMI和脂肪重量均有降低作用,且效果在超重/肥胖的成年人中比BMI正常的成年人更明显[19]。值得注意的是,肥胖人群血清L-肉碱浓度明显高于正常人群[20]。Heianza等[21]在510名超重和肥胖的受试者中发现,血清L-肉碱水平与BMI、躯干脂肪百分比和全身脂肪百分比呈正相关。实验室前期研究也发现,血清L-肉碱与成年女性肥胖程度成正比[22]。其原因可能是肥胖人群肌肉组织存储L-肉碱能力下降,其机制有待进一步探究。
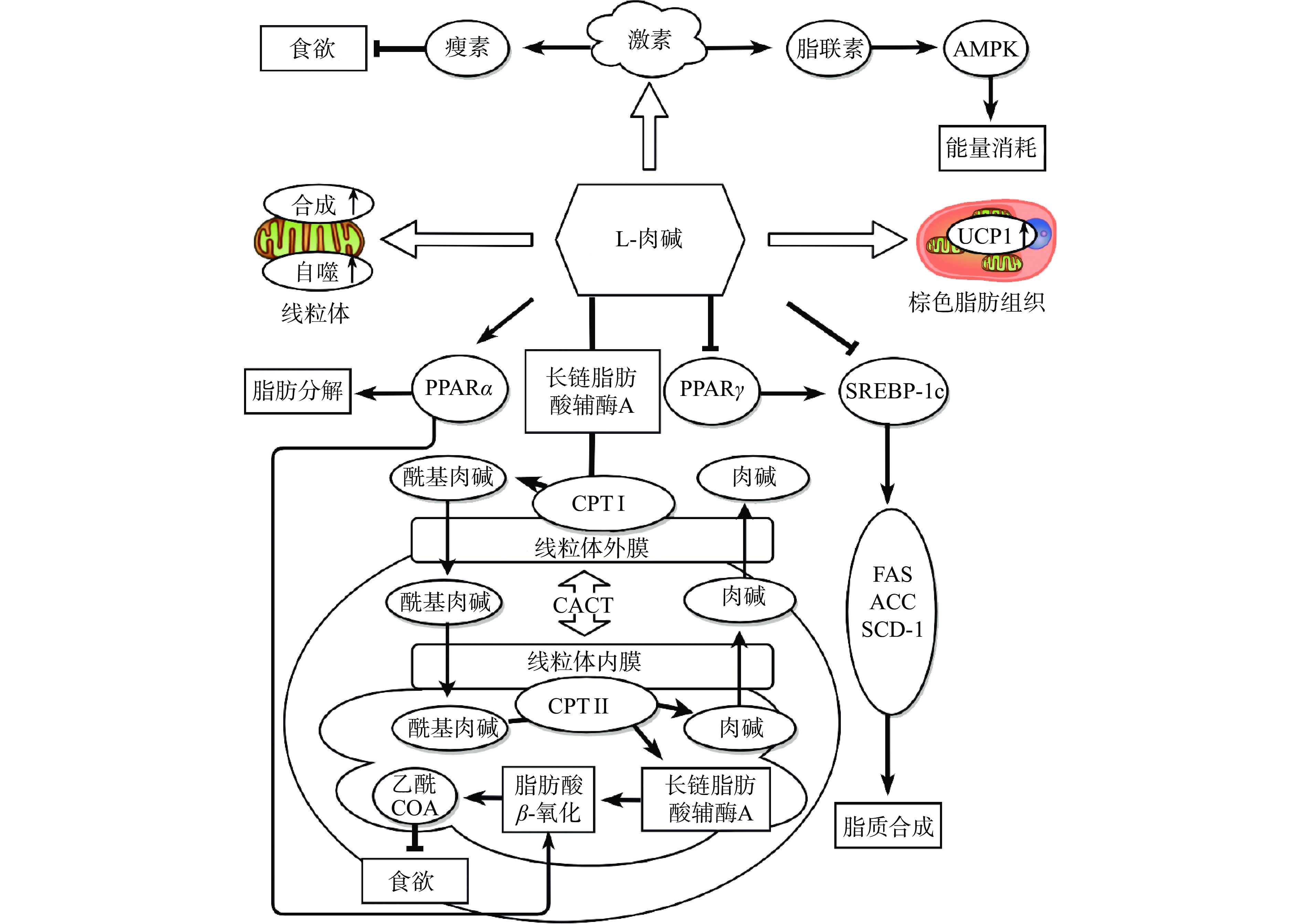
L-肉碱的减肥机制主要有以下几个方面(图3):a. L-肉碱作为脂肪酸β-氧化的强力辅助因子,可与长链酰基辅酶A在线粒体外膜外侧肉碱棕榈酰转移酶 Ⅰ(CPT Ⅰ)的催化下形成酰基肉碱,随后在肉碱酯酰转移酶(CACT)作用下将酰基肉碱跨过内膜输送至线粒体基质,最后在线粒体内膜内测CPT Ⅱ作用下重新分解为游离L-肉碱和酰基辅酶,促进后者在线粒体发生β-氧化[23]。b. L-肉碱可以调控多种脂质分解代谢或脂肪生成相关的转录因子,如过氧化物酶体增殖物激活受体(PPARs),固醇调节元件结合蛋白1c(SREBP-1c)等。PPAR作为调节脂质代谢的核受体和长链脂肪酸的配体在调节能量代谢中起重要作用[24]。PPAR-α是脂质分解代谢的关键调节因子。L-肉碱通过增加核受体与其启动子靶序列的结合亲和力,可上调PPAR-α mRNA的表达水平,促进脂肪分解和β-氧化[25]。此外,β氧化的最终产物乙酰辅酶A的增加,可影响大脑的葡萄糖供应,从而调节能量消耗,抑制食欲[26]。PPAR-γ在脂肪合成及脂肪细胞分化过程中发挥重要作用[27]。研究表明,L-肉碱可以在mRNA和蛋白水平上抑制PPAR-γ的表达[28-29]。SREBP-1c是另一重要的脂合成代谢相关转录因子,其表达同样受PPAR-γ调控[30]。通过激活SREBP-1c可开启脂质合成下游靶点,如乙酰辅酶A羧化酶(ACC)、脂肪酸合成酶(FAS)和脂肪酸转化酶(SCD-1),促进甘油三酯(TG)合成和脂质沉积[31]。L-肉碱可显著抑制高脂饮食诱导的肝脏SREBP-1c通路激活[32]。实验室前期研究也发现,L-肉碱可上调白色脂肪组织(WAT)中的PPARα并下调SREBP-1c的表达,阻止高脂饮食喂养诱导的雄性及雌性小鼠肥胖[18]。c. L-肉碱有助于维持褐色脂肪组织(BAT)形态及功能[33]。L-肉碱作为BAT中解偶联蛋白-1(UCP-1)介导下产生热量的必需化合物,可维持BAT典型细胞结构。肉碱缺乏的JVS小鼠BAT中脂滴过度积累[34]。d. L-肉碱有助于维持线粒体的功能。Cahova等[35]在L-肉碱治疗的HHTg大鼠观察到调节线粒体生物合成的转录因子(PGC1α,TFAM)表达增加,且mtDNA拷贝数增加,这表明L-肉碱刺激了HHTg大鼠肝脏中线粒体的形成。Choi等[36]在高脂饮食诱导的肥胖小鼠中发现,L-肉碱通过调节自噬显著改善肌肉组织中线粒体的功能紊乱。e. L-肉碱可调节脂肪组织瘦素和脂联素的分泌[37]。瘦素是一类脂源性蛋白类激素,作用于下丘脑,抑制食欲[38]。脂联素属于脂肪细胞因子中的保护性细胞因子。当脂联素与其受体结合后会导致腺苷酸活化蛋白激酶(AMPK)、PPAR-α和PPAR-γ等信号通路的激活[39],具有降低体重、抗炎及抑制血管形成等作用[40]。口服L-肉碱显著降低肥胖青少年血清瘦素浓度,升高脂联素浓度[41]。
综上,L-肉碱具有良好的减肥功效,且潜在分子机制挖掘较充足,表明其作为治疗、改善肥胖的营养补充剂或药品在食品、保健品及医药等领域具有广阔的应用前景。
3. 左旋肉碱与血脂异常
血脂异常是代谢综合征的重要特征之一,包括血清高密度脂蛋白-胆固醇(HDL-c)浓度下降,TG、胆固醇(TC)和低密度脂蛋白-胆固醇(LDL-c)水平升高。越来越多的证据表明L-肉碱对血脂异常具有改善作用。
动物实验研究表明,600 mg/kg L-肉碱腹腔注射8周可显著降低2型糖尿病大鼠血清总TC和LDL-c浓度[42]。高脂饮食的C57BL/6小鼠灌胃L-肉碱(500 mg/kg BW)25周后血清TG、TC、LDL-c/HDL-c比值均显著下降[43]。人群研究证据表明,24名年轻肥胖男性每天摄入3 g L-肉碱8周后血清TC、TG和LDL-c均显著下降,HDL-c显著升高[44]。每日2 g L-肉碱摄食补充可显著降低糖尿病患者血浆TG、LDL-c浓度[45]。在原发性肾病综合征患者中,2 g/d L-肉碱静脉注射8周后,血浆TC、TG及LDL-c水平显著下降,HDL-c水平显著升高[46]。2020年,一项基于8篇RCTs的meta分析表明,L-肉碱可有效降低2型糖尿病患者血液中TC和LDL-c的浓度,并且在干预周期超过12周的研究中,L-肉碱的降血脂效果更显著[47]。同年,一项基于24篇RCTs的meta分析结果提示,L-肉碱可显著降低心血管疾病患者血浆中TC、LDL-c并升高HDL-c的浓度,而对TG无显著作用[48]。由此可见,L肉碱对血脂具有明显的改善效果,尤其是降低TC、LDL-c的作用较为突出,而对TG及HDL-c的调节作用尚无定论。目前,尚无摄食补充L-肉碱对MS患者血脂调节作用的meta分析或综述性文章,有待更多的人群RCT研究加以验证。
L-肉碱改善血脂的可能机制有:a. L-肉碱参与脂肪酸代谢,促进脂肪酸β-氧化[49-50]。L-肉碱可以改变肝脏的脂代谢倾向,从甘油三酯的酯化和合成转向乙酰肉碱的形成,促进脂肪酸β-氧化,从而降低TG和LDL-c的合成。b. HDL-c在血清胆固醇清理中起积极作用,能将外周组织中多余的胆固醇带回肝脏进行代谢[51]。L-肉碱可以刺激HDL-c主要载脂蛋白载脂蛋白a1的产生,促进HDL-c的合成[52]。c. L-肉碱可以抑制胆固醇合成关键酶b-羟基b-甲基戊二酰(HMG)-CoA还原酶的活性,从而抑制胆固醇合成[53]。此外,胆汁酸合成、分泌增加会促进肝脏胆固醇分解代谢。L-肉碱上调小鼠肝脏胆汁酸合成限速基因胆固醇7α-羟化酶(Cyp7a1)的表达,促进肝脏胆固醇的分解[43]。d. L-肉碱调控AMPK-PPAR能量代谢通路。AMPK,即AMP依赖的蛋白激酶,是能量代谢的关键调节分子。其下游信号分子PPAR-α可调控甘油三酯脂肪酶(ATGL)和CPT Ⅰ的表达,促进甘油三酯水解和刺激长链脂肪酸从细胞质运输到线粒体基质进行脂肪酸β-氧化[54],从而促进能量消耗减少脂质生成。L-肉碱可能通过促进肝脏AMPK、PPAR-α的表达发挥降血脂作用[55]。
4. 左旋肉碱与糖代谢紊乱
糖代谢紊乱包括高血糖和IR,是MS的重要组成部分。IR更被认为是MS的中心环节及核心病理基础[56]。
高果糖高脂饲料诱导MS雄性SD大鼠中发现,L-肉碱(200 mg/kg)治疗4周可显著提高胰岛素敏感性,降低血糖浓度[57]。高脂饮食和链脲佐菌素注射联合诱导2型糖尿病雄性昆明小鼠每天灌胃125 mg/kg L-肉碱至12周龄,显示降低小鼠血糖水平,并升高血浆和胰腺中胰岛素样生长因子(IGF-1)浓度[58]。多项人群实验证据表明,L-肉碱干预对改善糖耐量和提高胰岛素敏感性具有显著作用。在一项辛伐他汀结合L-肉碱(规格:10 mL:1 g×6支)连续治疗3个月冠心病合并2型糖尿病患者中,观察组空腹血糖及餐后2 h血糖水平显著低于辛伐他汀对照组[59]。同样,在老年糖尿病合并心力衰竭患者中,2 g L-肉碱静脉滴注10 d后,患者餐后2 h血糖、空腹血糖均显著低于对照组[60]。超重/肥胖合并多囊软巢综合妇女每天摄食补充1000 mg L-肉碱12周,血清胰岛素浓度、HOMA-IR指数均相对于安慰剂组显著下降[61]。2021年,一项基于8篇RCTs的meta分析结果表明,每天补充2 g L-肉碱可以显著降低2型糖尿病患者空腹血糖及糖化血红蛋白浓度[62]。这些证据表明,L-肉碱具有潜在的胰岛素增敏功效,可能通过改善IR调节机体糖代谢紊乱、降低血糖。更多大型干预性临床研究的开展,有利于明确L-肉碱对糖代谢紊乱,尤其是胰岛素敏感性的调节作用。
L-肉碱调节糖代谢紊乱的机制尚不完全明确,可能通过以下机制调节血糖、改善胰岛素抵抗:首先,L-肉碱通过增加脂肪酸氧化,可以降低体内氧化应激,是其改善血糖的重要机制[49-50]。其次,L-肉碱还可以刺激IGF-1轴和磷脂酰肌醇-3-羟激酶/蛋白激酶B(PI3K/AKT)胰岛素信号通路[63],调节糖异生、糖酵解相关酶及葡萄糖转运体(GLUT)的表达,促进外周组织葡萄糖的利用并抑制糖异生[64]。再者,L-肉碱可抑制机体慢性炎症反应,抑制具有诱导胰岛素抵抗作用促炎症因子白介素-6(IL-6)、肿瘤怀死因子-α(TNF-α)等的分泌[65]。最后,L-肉碱可促进心脏葡萄糖的利用[64]。
5. 左旋肉碱与高血压
收缩压(SBP)≥140 mmHg和/或舒张压(DBP)≥90 mmHg[66]被定义为高血压,它也是MS的重要组成之一。动物实验结果提示,L-肉碱具有显著的降血压活性。高果糖喂养的Wistar大鼠腹腔注射L-肉碱(300 mg/kg)30 d显著降低了大鼠的血压[67]。同样,在自发性高血压(SHR)大鼠及Nω-硝基-l-精氨酸甲酯(L-NAME)诱导高血压大鼠中,300 mg/kg L-肉碱干预30 d显著降低了大鼠收缩压及舒张压[68]。人群研究方面,2019年,一项基于10篇RCTs的meta分析结果提示[69],摄食补充L-肉碱可以降低DBP,但不影响SBP水平,尤其在超重及肥胖人群中效果更显著。2020年,一篇RCT文章发现,50 mg/kg/d L-肉碱干预6个月显著降低了β地中海贫血患者的SBP[70]。同年,另一篇RCT文章结果表明,糖尿病患者口服L-肉碱1000 mg/d治疗12周对血压无明显影响[71]。由此可见,L-肉碱对血压的调节作用尚无法定论,尤其是人群研究的证据不能完全统一,有待更多基于高血压患者或MS患者的临床研究的开展加以验证。
L-肉碱的降血压机制尚不明确,目前已知的潜在机制有:a. 抑制血浆血管紧张素转化酶(ACE)活性。血浆ACE被认为是内皮细胞损伤的标志[72]。ACE催化无生物活性的血管紧张素 I转化为有活性的血管紧张素 II,从而引起血管平滑肌细胞收缩、血压升高。此外,ACE可抑制激肽酶-激肽系统,抑制具有血管舒张作用的舒缓激肽的释放。L-肉碱处理可显著降低高血压大鼠血浆及心脏的ACE的表达[73]。b. 促进一氧化氮(NO)的分泌,NO是血管内皮分泌的目前已知最强的血管舒张因子[74]。L-肉碱可显著升高高血压大鼠血清NO的浓度[75]。c. L-肉碱可以改善心肌细胞的能量代谢,提高心肌成纤维细胞的机械效率和功能,从而调节血压[76]。d. L-肉碱可降低血脂、缓解机体慢性炎症、改善肥胖及胰岛素抵抗,这些因素均是高血压的风险因素。
6. 左旋肉碱与高尿酸血症
高尿酸血症是MS的重要组分之一。MS患者,血清尿酸水平明显升高[77]。目前,少量研究表明补充L-肉碱具有潜在降尿酸作用。高脂喂养16周Wistar大鼠,继续给予1.2% L-肉碱饮食干预8周后血清尿酸浓度显著下降[78]。氧嗪酸诱导高尿酸大鼠腹腔注射500 mg/kg/d L-肉碱4周后高尿酸血症明显改善[79]。同样,饮水添加0.1% L-肉碱4周显著降低自发性高血压大鼠血尿酸浓度[80]。迄今为止,L-肉碱与尿酸关系的人群研究仍较少。在26例残疾青少年中观察到血清L-肉碱与尿酸浓度显著正相关[81]。30例慢性心力衰竭患者静脉注射2 g L-肉碱7 d后血清尿酸浓度显著低于对照组[82]。15名接受禁食干预的MS患者静脉注射4 g/d L-肉碱7 d血清尿酸浓度相对于未进行L-肉碱干预的患者无明显差异[83]。20名健康的老年妇女连续每天摄食补充1500 mg L-肉碱24周,血清尿酸浓度无明显变化[84]。目前,尚无L-肉碱干预对痛风病人或高尿酸血症患者尿酸水平调节作用的报道。要明确L-肉碱对高尿酸血症的影响,将依赖更多经过严格控制且纳入足够多受试者的RCT试验加以证明。
L-肉碱调节尿酸的机制研究较少,现有的证明提示L-肉碱可能通过其抗炎、抗肝脏脂肪变性和抗氧化功能,发挥降尿酸活性[79]。高尿酸血症是由于尿酸生成过剩(占高尿酸血症病例的10%)或者尿酸排泄减少造成的(90%的案例)[85]。尿酸是一种弱酸性的阴离子,有机阴离子转运蛋白1(OAT1)和OAT3参与了尿酸的排泄过程[86]。高尿酸血症大鼠肾脏OAT1和OAT3表达显著减少[87]。目前,尚无研究证明L-肉碱是否调节了尿酸的合成代谢或排泄过程,有待更多的体内及体外实验进行探讨。
7. 左旋肉碱与氧化三甲胺
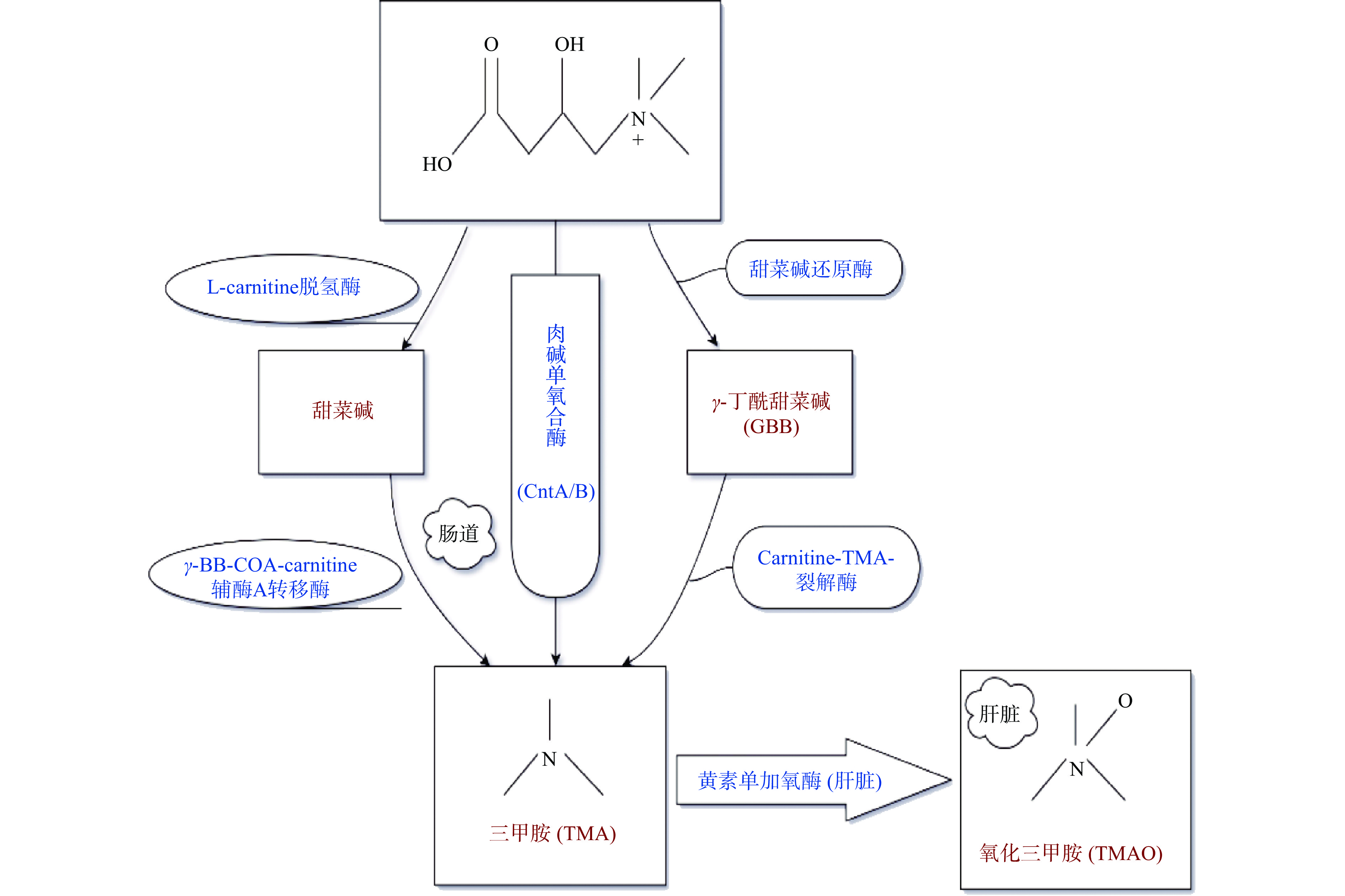
氧化三甲胺(TMAO)是三甲胺(TMA)的氧化产物,分子式为(CH3)3NO。近年,TMAO作为心血管疾病的独立风险因子已被人们熟知,在多种代谢性疾病,包括心血管疾病、2型糖尿病、慢性肾脏疾病、高血压、肥胖、阿尔兹海默症等患者中均观察到血清TMAO浓度升高[88]。TMAO对MS同样存在不利影响。健康个体血浆TMAO浓度为0.5~5.0 μmol/L,TMAO浓度升高会显著增加MS的风险[89]。血清中TMAO浓度值≥ 8.74 µmol/L被认为是MS的预测因子[90]。动物实验证明,摄食补充TMAO可加剧MS的核心胰岛素抵抗[91]。L-肉碱是TMAO的重要前体物质之一。摄食L-肉碱可显著增加循环系统TMAO的浓度[92]。L-肉碱摄入后,不能被小肠完全吸收,从食物中获取的L-肉碱吸收率约为54%~86%[93],而通过口服补充的L-肉碱只有5%~25%[94]。在肠道中不被吸收的L-肉碱,可通过肠道微生物肉碱单氧合酶(Cnt)A/B作用可直接转化成TMA,也可通过其他酶转化成另外两种TMA前体物质,如甜菜碱(通过左旋肉碱脱氢酶)和γ-丁酰甜菜碱(GBB)(通过甜菜碱还原酶)[10]。小肠(空肠,尤其是回肠)是L-肉碱产生GBB和甜菜碱的主要起始部位。由L-肉碱和GBB以及甜菜碱产生TMA的主要位点在富含细菌的盲肠(大肠的起始部位)[95]。随后这些代谢中间体在大肠中具有肉碱-TMA裂解酶的肠道微生物如Acinetobacter的作用下,C-N键断裂,代谢为TMA,或者在γ-BB-COA肉碱辅酶A转移酶的作用下生成TMA,之后大部分TMA(近95 %)会迅速通过血管壁进入循环系统[92]。TMA通过门静脉血到达肝脏后,在肝脏经黄素单氧化酶(FMOs)氧化成TMAO[92](图4)。其中,FMO3是人体及哺乳动物肝脏氧化TMA的关键酶[92]。TMA被氧化成TMAO后重新进入血液循环运往全身组织作为渗透压调节剂积累,或者被肾脏清除排泄[88],在24小时内以TMA:TMAO(3:95)的比例随尿液排出。TMAO还可通过汗液、粪便(4%)、呼出的空气(不到1%)或其他身体分泌物等被排泄[96]。
最新研究表明,L-肉碱由于代谢产生TMAO或可对MS带来不利影响。摄食L-肉碱可通过代谢产生TMAO促进ApoE敲除小鼠动脉粥样硬化的进程[92]。大剂量L-肉碱(3%)干预引起的小鼠肝功能损伤可能与TMAO的产生相关[97]。然而,也有文章表明摄食L-肉碱虽显著升高了血浆TMAO的浓度,但未对心血管疾病的相关指标造成不利影响[98]。TMAO的产生增加了L-肉碱与健康关系的不确定性。目前,人群研究方面尚无补充L-肉碱由于代谢产生TMAO带来健康风险的直接证据。推测,可能是由于L-肉碱本身具有良好的生物活性,可以对抗其内源性产生TMAO带来的不利影响。大型前瞻性队列研究或长期L-肉碱干预的随机临床对照实验的开展,将有利于进一步揭示L-肉碱、TMAO及MS的关系。
8. 结语
L-肉碱具有良好的生物活性,在MS的防治中受到越来越多的关注。本文详细论述了L-肉碱与MS各组分关系的研究进展,并对其作用机制进行了总结。L-肉碱作为调节脂代谢的关键成分对肥胖、血脂异常的改善作用证据充足,表现出良好的效果。在改善高血糖,提高胰岛素敏感性方面,L-肉碱同样表现出显著的活性,且人群证据较为充足。L-肉碱对高血压、高尿酸血症的调节作用结论尚无法统一,虽然在动物实验方面L-肉碱对两种症状均表现出良好的改善效果,但人群研究的证据较少,缺乏合适的人群模型及大型的随机临床对照试验的数据支撑,有待进一步明确。此外,L-肉碱对MS相关指标的改善机制虽然进行了大量工作,但其具体的作用靶点仍有待明确,尤其是其降血压、降尿酸的机制研究缺乏,需进一步探究。
L-肉碱的食物来源广泛且人体可自身合成,可满足成人的基本需要,目前中国营养协会等组织对L-肉碱的RNI、AI及UL等也无相应的膳食补充参考建议。L-肉碱作为营养补充剂具有较好的安全性,但近年,由于其经肠道代谢产生TMAO的问题,L-肉碱摄入的潜在健康风险引起了新的关注。目前并无直接的人群证据表明,摄食补充L-肉碱由于代谢产生了TMAO对MS带来不利影响。L-肉碱摄食的安全性问题有待大型前瞻性队列研究或长期随机临床对照试验加以验证。
-
[1] LEMIEUX I, DESPRÉS J. Metabolic syndrome: Past, present and future[J]. Nutrients,2020,12(11):3501−3507. doi: 10.3390/nu12113501
[2] SAKLAYEN M G. The global epidemic of the metabolic syndrome[J]. Current Hypertension Reports,2018,20(2):12−20. doi: 10.1007/s11906-018-0812-z
[3] 中国心血管健康与疾病报告编写组. 中国心血管健康与疾病报告2019概要[J]. 心脑血管病防治,2020,20(5):437−450. [Chinese Cardiovascular Health and Disease Report Writing Group. Report on cardiovascular health and diseases in China 2019: An updated summary[J]. Prevention and Treatment of Cardio-Cerebral-Vascular Disease,2020,20(5):437−450. doi: 10.3969/j.issn.1009-816x.2020.05.001 [4] CHOI M J, PARK S M, LEE M S. L-carnitine's effect on the biomarkers of metabolic syndrome: A systematic review and meta-analysis of randomized controlled trials[J]. Nutrients,2020,12(9):2795−2807. doi: 10.3390/nu12092795
[5] 胡祥勇, 王虹剑. 口服左旋肉碱和动脉粥样硬化风险相关性的研究进展[J]. 中国分子心脏病学杂志,2020,20(3):3421−3424. [HU X Y, WANG H J. Study advance on the correlation between the metabolism of L-carnitine through intestinal microorganisms and the risk of atherosclerosis[J]. Molecular Cardiology of China,2020,20(3):3421−3424. doi: 10.16563/j.cnki.1671-6272.2020.06.017 [6] REUTER S E, EVANS A M. Carnitine and acylcarnitines: Pharmacokinetic, pharmacological and clinical aspects[J]. Clinical Pharmacokinetics,2012,51(9):553−572. doi: 10.1007/BF03261931
[7] ADEVA-ANDANY M M, CALVO-CASTRO I, FERNÁNDEZ-FERNÁNDEZ C, et al. Significance of L-carnitine for human health[J]. IUBMB Life,2017,69(8):578−594. doi: 10.1002/iub.1646
[8] DEMARQUOY J, GEORGES B, RIGAULT C, et al. Radioisotopic determination of L-carnitine content in foods commonly eaten in western countries[J]. Food Chemistry,2003,86(1):137−142.
[9] ÖZOGUL Y, KULEY B E, ÖZOGUL F, et al. L-carnitine contents in seafoods commonly eaten in middle eastern countries[J]. Journal of Food Biochemistry,2012,37(6):702−707.
[10] KOETH R A, LAM-GALVEZ B R, KIRSOP J, et al. L-carnitine in omnivorous diets induces an atherogenic gut microbial pathway in humans[J]. The Journal of Clinical Investigation,2019,129(1):373−387.
[11] 郑鹏然. 肉碱功能及其安全性评价(综述)[J]. 中国食品卫生杂志,1993(4):52−54. [ZHENG P R. Function and safety evaluation of carnitine (review)[J]. Chinese Journal of Food Hygiene,1993(4):52−54. doi: 10.13590/j.cjfh.1993.04.027 [12] ALESCIIRINI S, COSTELLO M R, COATES P, et al. Preface: Carnitine: Lessons from one hundred years of research[J]. Annals of the New York Academy of Sciences,2010:1033−1037.
[13] HATHCOCK J N, SHAO A. Risk assessment for carnitine[J]. Regulatory Toxicology and Pharmacology,2006,46(1):23−28. doi: 10.1016/j.yrtph.2006.06.007
[14] FRITZ I B, MCEWEN B. Effects of carnitine on fatty-acid oxidation by muscle[J]. Science,1959,129(3345):334−335. doi: 10.1126/science.129.3345.334
[15] ESMAIL M, ANWAR S, KANDEIL M, et al. Effect of nigella sativa, atorvastatin, or L-carnitine on high fat diet-induced obesity in adult male albino rats[J]. Biomedicine & Pharmacotherapy,2021,141:111818−111826.
[16] TRKAY B K, BAALP A. Effect of L-carnitine used in regular exercise in elderly obesis rats[J]. Turkish Journal of Sport and Exercise,2019,21(1):74−77.
[17] MUN E G, SOH J R, CHA Y S. L-carnitine reduces obesity caused by high-fat diet in C57BL/6J mice[J]. Food Science and Biotechnology,2007,16(2):228−233.
[18] GAO X, SUN G, RANDELL E, et al. Systematic investigation of the relationships of trimethylamine N-oxide and L-carnitine with obesity in both humans and rodents[J]. Food & Function,2020:1039−1052.
[19] ASKARPOUR M, HADI A, MIRAGHAJANI M, et al. Beneficial effects of L-carnitine supplementation for weight management in overweight and obese adults: An updated systematic review and dose-response meta-analysis of randomized controlled trials[J]. Pharmacological Research,2020,151(C):104554−104590.
[20] ZHAO Y, YANG N, GAO J, et al. The effect of different L-carnitine administration routes on the development of atherosclerosis in ApoE knockout mice[J]. Molecular Nutrition & Food Research,2018,62(5):1700299−1700329.
[21] HEIANZA Y, SUN D, SMITH S R, et al. Changes in gut microbiota-related metabolites and long-term successful weight loss in response to weight-loss diets: The pounds lost trial[J]. Circulation,2018,41(3):413−419.
[22] GAO X, TIAN Y, RANDELL E, et al. Unfavorable associations between serum trimethylamine N-oxide and L-carnitine levels with components of metabolic syndrome in the Newfoundland population[J]. Frontiers in Endocrinology,2019,10:168−196. doi: 10.3389/fendo.2019.00168
[23] WANG J M, XIANG H J, LU Y F, et al. The role and therapeutic implication of CPTs in fatty acid oxidation and cancers progression[J]. American Journal of Cancer Research,2021,11(6):2477−2494.
[24] SANJAY, SHARMA A, LEE H J. Role of phytoconstituents as PPAR agonists: Implications for neurodegenerative disorders[J]. Biomedicines,2021,9(12):1914−1950. doi: 10.3390/biomedicines9121914
[25] FRSTER L, INDRA D, KIENESBERGER K, et al. L-carnitine exerts a nutrigenomic effect via direct modulation of nuclear receptor signaling in adipocytes, hepatocytes, and skmc demonstrating its nutritional impact[J]. Nutrition Research,2020,85:84−98.
[26] KIM J H, PAN J H, LEE E S, et al. L-carnitine enhances exercise endurance capacity by promoting muscle oxidative metabolism in mice[J]. Biochemical and Biophysical Research Communications,2015,464(2):568−573. doi: 10.1016/j.bbrc.2015.07.009
[27] MAL S, DWIVEDI A R, KUMAR V, et al. Role of peroxisome proliferator-activated receptor gamma (PPARγ) in different disease states: Recent updates[J]. Current Medicinal Chemistry,2021,28(16):3193−3215. doi: 10.2174/0929867327666200716113136
[28] BING C, RUSSELL S, BECKET E, et al. Adipose atrophy in cancer cachexia: Morphologic and molecular analysis of adipose tissue in tumour-bearing mice[J]. British Journal of Cancer,2006,95(8):1028−1037. doi: 10.1038/sj.bjc.6603360
[29] BATISTA M L, NEVES R X, PERES S B, et al. Heterogeneous time-dependent response of adipose tissue during the development of cancer cachexia[J]. Journal of Endocrinology,2012,215(3):363−373. doi: 10.1530/JOE-12-0307
[30] FERRÉ P, PHAN F, FOUFELLE F. SREBP-1c and lipogenesis in the liver: An update1[J]. The Biochemical Journal,2021,478(20):3723−3739. doi: 10.1042/BCJ20210071
[31] HU Y Y, YIN F W, LIU Z Y, et al. Acerola polysaccharides ameliorate high-fat diet-induced non-alcoholic fatty liver disease through reduction of lipogenesis and improvement of mitochondrial functions in mice[J]. Food & Function,2020,11(1):1037−1048.
[32] KON K, IKEJIMA K, MORINAGA M, et al. L-carnitine prevents metabolic steatohepatitis in obese diabetic kk-ay mice[J]. Hepatology Research,2017,47(3):E44−E54. doi: 10.1111/hepr.12720
[33] OZAKI K, SANO T, TSUJI N, et al. Carnitine is necessary to maintain the phenotype and function of brown adipose tissue[J]. Laboratory Investigation,2011,91(5):704−710. doi: 10.1038/labinvest.2011.6
[34] NARAMA I, OZAKI K, MATSUURA T, et al. Histopathology of the congenitally carnitine-deficient JVS mouse[J]. BioMed Research International,1997,84(6):851−857.
[35] CAHOVA M, CHRASTINA P, HANSIKOVA H, et al. Carnitine supplementation alleviates lipid metabolism derangements and protects against oxidative stress in non-obese hereditary hypertriglyceridemic rats[J]. Applied Physiology, Nutrition, and Metabolism,2015,40(3):280−291. doi: 10.1139/apnm-2014-0163
[36] CHOI J W, OHN J H, JUNG H S, et al. Carnitine induces autophagy and restores high-fat diet-induced mitochondrial dysfunction[J]. Metabolism,2018,78:43−51. doi: 10.1016/j.metabol.2017.09.005
[37] TIENHOVEN-WIND L V, DULLAART R P F. Increased leptin/adiponectin ratio relates to low-normal thyroid function in metabolic syndrome[J]. Lipids in Health and Disease,2017,16(1):1−6. doi: 10.1186/s12944-016-0392-3
[38] MUHAMMAD W, RABBI A F, SADIA N S, et al. Role of leptin deficiency, inefficiency, and leptin receptors in obesity[J]. Biochemical Genetics,2016,54(5):565−572. doi: 10.1007/s10528-016-9751-z
[39] KALKMAN H O. An explanation for the adiponectin paradox[J]. Pharmaceuticals (Basel),2021,14(12):1266−1274. doi: 10.3390/ph14121266
[40] WANG Z V, SCHERER P E. Adiponectin, the past two decades[J]. Journal of Molecular Cell Biology,2016,8(2):93−100. doi: 10.1093/jmcb/mjw011
[41] 杨娜. 左卡尼汀对体质量指数≥32 kg/m2单纯性肥胖青少年血清脂联素及肝脏功能的影响[J]. 中国药业,2018,27(9):32−34. [YANG N. Effect of levocarnitine on serum adiponectin and liver function in simple obesity adolescents with BMI≥32 kg/m2[J]. China Pharmaceuticals,2018,27(9):32−34. doi: 10.3969/j.issn.1006-4931.2018.09.010 [42] SALMANOGLU D S, GURPINAR T, VURAL K, et al. Melatonin and L-carnitin improves endothelial disfunction and oxidative stress in type 2 diabetic rats[J]. Redox Biology,2016,8:199−204. doi: 10.1016/j.redox.2015.11.007
[43] SU C C, CHANG C S, CHOU C H, et al. L-carnitine ameliorates dyslipidemic and hepatic disorders induced by a high-fat diet via regulating lipid metabolism, self-antioxidant capacity, and inflammatory response[J]. Journal of Functional Foods,2015,15:497−508. doi: 10.1016/j.jff.2015.04.007
[44] HAKIMI M, SHEIKHOLESLAMI-VATANI D, ALI-MOHAMMADI M. The effect of concurrent training combined with ingested of L-carnitine supplementation on hormonal changes, lipid profile and body composition in obese men[J]. Mini-Reviews in Organic Chemistry,2015,26(3):185−193.
[45] MALAGUARNERA M, VACANTE M, AVITABILE T, et al. L-carnitine supplementation reduces oxidized LDL cholesterol in patients with diabetes[J]. The American Journal of Clinical Nutrition,2009,89(1):71−76. doi: 10.3945/ajcn.2008.26251
[46] YANG B. Effect of L-carnitine on PNS, lipid metabolism and oxidative stress indicators[J]. Medical Journal of National Defending Forces in Southwest China,2019,29(1):30−33.
[47] ASBAGHI O, KASHKOOLI S, AMINI M R, et al. The effects of L-carnitine supplementation on lipid concentrations in patients with type 2 diabetes: A systematic review and meta-analysis of randomized clinical trials[J]. Journal of Cardiovascular and Thoracic Research,2020,12(4):246−255. doi: 10.34172/jcvtr.2020.43
[48] ASADI M, RAHIMLOU M, SHISHEHBOR F, et al. The effect of L-carnitine supplementation on lipid profile and glycaemic control in adults with cardiovascular risk factors: A systematic review and meta-analysis of randomized controlled clinical trials[J]. Clinical Nutrition,2020,39(1):110−122. doi: 10.1016/j.clnu.2019.01.020
[49] LI J M, LI L Y, QIN X, et al. Systemic regulation of L-carnitine in nutritional metabolism in zebrafish, danio rerio[J]. Scientific Reports,2017,7:40815−40828. doi: 10.1038/srep40815
[50] OZORIO R O A, GINNEKEN V J T V, BESSA R J B, et al. Effects of exercise on L-carnitine and lipid metabolism in African catfish (Clarias gariepinus) fed different dietary L-carnitine and lipid levels[J]. British Journal of Nutrition,2009,103(8):1139−1150.
[51] KARDASSIS D, THYMIAKOU E, CHRONI A. Genetics and regulation of HDL metabolism[J]. Biochim Biophys Acta Mol Cell Biol Lipid,2022,1867(1):159060−1590609.
[52] GIROLAMI A, SARTORI M T, LOMBARDI M A, et al. Metabolic effects of L-carnitine on type 2 diabetes mellitus: Systematic review and meta-analysis[J]. Experimental and Clinical Endocrinology & Diabetes,2013,121(4):234−238.
[53] MONDOLA P, SANTILLO M, MERCATO R D, et al. The effect of L-carnitine on cholesterol metabolism in rat (Rattus bubalus) hepatocyte cells[J]. International Journal of Biochemistry,1992,24(7):1047−1050. doi: 10.1016/0020-711X(92)90372-8
[54] DUBOIS V, EECKHOUTE J, LEFEBVRE P, et al. Distinct but complementary contributions of ppar isotypes to energy homeostasis[J]. The Journal of Clinical Investigation,2017,127(4):1202−1214. doi: 10.1172/JCI88894
[55] FU Y. Effect of L-carnitine and exercise on fat-rich dietmice pparα and lpl in liver[J]. Medicine & Science in Sports & Exercise,2020,52(7S):1080−1081.
[56] TONG Y, XU S, HUANG L, et al. Obesity and insulin resistance: Pathophysiology and treatment[J]. Drug Discovery Today,2021(21):479−485.
[57] ZAYED E A, AINSHOKA A A, EL S K A, et al. Improvement of insulin resistance via increase of GLUT4 and PPARγ in metabolic syndrome-induced rats treated with omega-3 fatty acid or L-carnitine[J]. Journal of Biochemical and Molecular Toxicology,2018,32(11):e22218−e22223.
[58] WANG R, WANG L X, ZHANG C S, et al. L-carnitine ameliorates peripheral neuropathy in diabetic mice with a corresponding increase in insulin-like growth factor-1 level[J]. Molecular Medicine Reports,2019,19(1):743−751.
[59] 林坚. 辛伐他汀结合左卡尼汀治疗冠心病合并2型糖尿病的临床效果观察[J]. 吉林医学,2019,40(2):259−261. [LIN J. Clinical efficacy of simvastatin combined with L-carnitine in treatment of coronary heart disease complicated with type 2 diabetes mellitus[J]. Jilin Medical Journal,2019,40(2):259−261. doi: 10.3969/j.issn.1004-0412.2019.02.023 [60] CHEN Y, LIU S. The effect of L-carnitine in the treatment of diabetes mellitus complicated with heart failure[J]. China Continuing Medical Education,2019,11(6):114−116.
[61] SANGOUNI A A, PAKRAVANFAR F, GHADIRI-ANARI A, et al. The effect of L-carnitine supplementation on insulin resistance, sex hormone-binding globulin and lipid profile in overweight/obese women with polycystic ovary syndrome: A randomized clinical trial[J]. European Journal of Nutrition,2021,62:1−13.
[62] WANG D D, MAO Y Z, HE S M, et al. Quantitative efficacy of L-carnitine supplementation on glycemic control in type 2 diabetes mellitus patients[J]. Expert Review of Clinical Pharmacology,2021,14(7):919−926. doi: 10.1080/17512433.2021.1917381
[63] ALISON S A, JANOS K A. CHARLES L H. Carnitine: A nutritional, biosynthetic, and functional perspective[J]. Molecular Aspects of Medicine, 2004, 25(5–6): 455-473.
[64] RINGSEIS R, KELLER J, EDER K. Role of carnitine in the regulation of glucose homeostasis and insulin sensitivity: Evidence from in vivo and in vitro studies with carnitine supplementation and carnitine deficiency[J]. European Journal of Nutrition,2012,51(1):1−18. doi: 10.1007/s00394-011-0284-2
[65] HALLAJZADEH J. The effects of L-carnitine supplementation on indicators of inflammation and oxidative stress: A systematic review and meta-analysis of randomized controlled trials[J]. Journal of Diabetes and Metabolic Disorders,2020,19(2):120277−120282.
[66] SILVA A A D, CARMO J M D, LI X, et al. Role of hyperinsulinemia and insulin resistance in hypertension: Metabolic syndrome revisited[J]. Canadian Journal of Cardiology,2020,36(5):671−682. doi: 10.1016/j.cjca.2020.02.066
[67] RAJASEKAR P, PALANISAMY N, ANURADHA C V. Increase in nitric oxide and reductions in blood pressure, protein kinase c beta ii and oxidative stress by L-carnitine: A study in the fructose-fed hypertensive rat[J]. Clinical and Experimental Hypertension,2007,29(8):517−546. doi: 10.1080/10641960701743998
[68] MATE A, MIGUEL-CARRASCO J L, MONSERRAT M T, et al. Systemic antioxidant properties of L-carnitine in two different models of arterial hypertension[J]. Journal of Physiology and Biochemistry,2010,66(2):127−136. doi: 10.1007/s13105-010-0017-7
[69] ASKARPOUR M, HADI A, BOZORG A, et al. Effects of L-carnitine supplementation on blood pressure: A systematic review and meta-analysis of randomized controlled trials[J]. Journal of Human Hypertension,2019,33(1-S):1−10.
[70] SHAHIDI M, HASHEMI S R, FATTAHI N, et al. The effects of L-carnitine on echocardiographic changes in patients with β-thalassemia major and intermedia[J]. Journal of Pediatric Hematology/Oncology,2020,42(6):386−390. doi: 10.1097/MPH.0000000000001850
[71] TALENEZHAD N, HOSSEINZADEH M, RAHMANIAN M, et al. The effect of L-carnitine supplementation on blood pressure in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial[J]. Obesity Medicine,2020,18:15−19.
[72] GIANI J F, VEIRAS L C, SHEN J Z Y, et al. Novel roles of the renal angiotensin-converting enzyme[J]. Molecular and Cellular Endocrinology,2021,529:1111257−111263.
[73] ARIAS J. The role of inflammatory markers in the cardioprotective effect of L-carnitine in L-NAME-induced hypertension[J]. American Journal of Hypertension,2008,21(11):1231−1237. doi: 10.1038/ajh.2008.271
[74] 邓林华, 温玉, 张运娇, 等. 血管内皮功能障碍与高血压关系的研究进展[J]. 中华高血压杂志,2021,29(10):935−940. [DENG L H, WEN Y, ZHANG Y J, et al. Research progress on the relationship between vascular endothelial dysfunction and hypertension[J]. Chinese Journal of Hypertension,2021,29(10):935−940. doi: 10.16439/j.issn.1673-7245.2021.10.008 [75] BUENO R, SOTOMAYOR M, PEREZ-GUERRERO C, et al. L-carnitine and propionyl-L-carnitine improve endothelial dysfunction in spontaneously hypertensive rats: Different participation of no and cox-products[J]. Life Sciences,2005,77(17):2082−2097. doi: 10.1016/j.lfs.2005.01.035
[76] SONG X, QU H, YANG Z, et al. Efficacy and safety of L-carnitine treatment for chronic heart failure: A meta-analysis of randomized controlled trials[J]. BioMed Research International,2017,4:6274854−6274865.
[77] İNANIR M. Serum uric acid (SUA) in morbidly obese patients and its relationship with metabolic syndrome[J]. Aging Male,2020,23(5):1165−1169. doi: 10.1080/13685538.2020.1713742
[78] PANCHAL S K, POUDYAL H, WARD L C, et al. Modulation of tissue fatty acids by L-carnitine attenuates metabolic syndrome in diet-induced obese rats[J]. Food Funct,2015,6(8):2496−2506. doi: 10.1039/C5FO00480B
[79] EL-KAFOURY B M A, AHMED M A, HAMMOUDA G A, et al. Possible role of L-carnitine in improvement of metabolic and hepatic changes in hyperuricemic and hyperuricemic-fructose-supplemented rats[J]. Physiological Reports,2019,7(22):e14282−e14293.
[80] RAUCHOVÁ H, DOBEšOVÁ Z, DRAHOTA Z, et al. The effect of chronic L-carnitine treatment on blood pressure and plasma lipids in spontaneously hypertensive rats[J]. European Journal of Pharmacology,1998,342(2):235−239.
[81] TAKEDA Y, KUBOTA M, SATO H, et al. Carnitine in severely disabled patients: Relation to anthropometric, biochemical variables, and nutritional intake[J]. Brain & Development,2015,37(1):94−100.
[82] 古思嘉, 徐广, 魏晓, 等. 左卡尼汀在治疗慢性心力衰竭中血尿酸浓度临床观察[J]. 中国医药导刊,2015,17(7):697−698. [GU S J, XU G, WEI X, et al. Clinical observation of serum uric acid concentration on L-carnitine in the treatment of chronic heart failure[J]. Chinese Journal of Medical Guide,2015,17(7):697−698. doi: 10.3969/j.issn.1009-0959.2015.07.026 [83] ZHANG J J, WU Z B, CAI Y J, et al. L-carnitine ameliorated fasting-induced fatigue, hunger, and metabolic abnormalities in patients with metabolic syndrome: A randomized controlled study[J]. Nutrition Journal,2014,13(1):1−11. doi: 10.1186/1475-2891-13-1
[84] OLEK R A, SAMULAK J J, SAWICKA A K, et al. Increased trimethylamine N-oxide is not associated with oxidative stress markers in healthy aged women[J]. Oxidative Medicine and Cellular Longevity,2019:6247169−6247176.
[85] CHEN X M, YOKOSE C, RAI S K, et al. Contemporary prevalence of gout and hyperuricemia in the United States and decadal trends: The national health and nutrition examination survey, 2007-2016[J]. Arthritis Rheumatol,2019,71(6):991−999. doi: 10.1002/art.40807
[86] ERALY S A, VALLON V, RIEG T, et al. Multiple organic anion transporters contribute to net renal excretion of uric acid[J]. Physiological Genomics,2008,33(2):180−192. doi: 10.1152/physiolgenomics.00207.2007
[87] HABU Y, YANO I, TAKEUCHI A, et al. Decreased activity of basolateral organic ion transports in hyperuricemic rat kidney: Roles of organic ion transporters, roat1, roat3 and roct2[J]. Biochemical Pharmacology,2003,66(6):1107−1114. doi: 10.1016/S0006-2952(03)00466-0
[88] SUBRAMANIAM S, FLETCHER C. Trimethylamine n-oxide: Breathe new life[J]. British Journal of Pharmacology,2018,175(8):1344−1353. doi: 10.1111/bph.13959
[89] HUSSEIN A R, GIULIA P, JUAN-CARLOS K D, et al. The role of microbiota in cardiovascular risk: Focus on trimethylamine oxide[J]. Current Problems in Cardiology,2019,44(6):182−196. doi: 10.1016/j.cpcardiol.2018.06.005
[90] LUIGI B, GIUSEPPE A, GIOVANNA M, et al. Trimethylamine n-oxide, mediterranean diet, and nutrition in healthy, normal-weight adults: Also a matter of sex?[J]. Nutrition (Burbank, Los Angeles County, Calif. ),2019,62:7−17. doi: 10.1016/j.nut.2018.11.015
[91] CHEN S, HENDERSON A, PETRIELLO M C, et al. Trimethylamine N-oxide binds and activates PERK to promote metabolic dysfunction[J]. Cell Metabolism,2019,30(6):1141−1151. doi: 10.1016/j.cmet.2019.08.021
[92] KOETH R A, WANG Z, LEVISON B S, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis[J]. Nature Medicine,2013,19(5):576−585. doi: 10.1038/nm.3145
[93] EVANS A M, FORNASINI G. Pharmacokinetics of L-carnitine[J]. Clinical Pharmacokinetics,2003,42(11):941−967. doi: 10.2165/00003088-200342110-00002
[94] REBOUCHE C, PAULSON D. Carnitine metabolism and function in humans[J]. Annual Review of Nutrition,1986,6:41−66. doi: 10.1146/annurev.nu.06.070186.000353
[95] KOETH R, LEVISON B, CULLEY M, et al. Γ-butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to tmao[J]. Cell Metabolism,2014,20(5):799−812. doi: 10.1016/j.cmet.2014.10.006
[96] PAPANDREOU C, MORÉ M, BELLAMINE A. Trimethylamine N-oxide in relation to cardiometabolic health-cause or effect?[J]. Nutrients,2020,12(5):1330−1365. doi: 10.3390/nu12051330
[97] WU Q, ZHANG X, ZHAO Y, et al. High L-carnitine ingestion impairs liver function by disordering gut bacteria composition in mice[J]. Journal of Agricultural and Food Chemistry,2020,68(20):5707−5714. doi: 10.1021/acs.jafc.9b08313
[98] SAMULAK J J, SAWICKA A K, HARTMANE D, et al. L-carnitine supplementation increases trimethylamine-N-oxide but not markers of atherosclerosis in healthy aged women[J]. Annals of Nutrition and Metabolism,2019,74(1):11−17. doi: 10.1159/000495037





 下载:
下载:
 下载:
下载:
