Optimization of High Yield Conditions and Antioxidant Activity of Ganoderma lingzhi Exopolysaccharide Promoted by Vernonia amygdalina Leaf
-
摘要: 胞外多糖(EPS)是灵芝液体发酵的主要活性产物,为提高灵芝胞外多糖含量,在发酵培养基中添加扁桃斑鸠菊叶粉末,采用单因素和正交试验优化发酵条件,红外光谱表征灵芝多糖结构,并分析其抗氧化活性。单因素实验结果表明,扁桃斑鸠菊叶粉末优化的添加量为4 g/L,胞外多糖含量与对照相比提高到167%;正交试验优化的发酵条件为:发酵时间12 d、起始pH5.0、转速120 r/min、扁桃斑鸠菊叶粉末的添加量为4 g/L,在此条件下,胞外多糖含量达到 13.05 g/L;红外光谱图表明添加和未添加扁桃斑鸠菊叶粉末的灵芝胞外多糖类型相似;抗氧化活性实验显示扁桃斑鸠菊叶粉末添加对胞外多糖清除ABTS+·的影响较小,但增强了其对·OH、DPPH·清除能力和Fe3+还原能力。扁桃斑鸠菊叶能有效提高灵芝胞外多糖的含量,为灵芝多糖的高效生产提供新思路。Abstract: Exopolysaccharide (EPS) is the main active metabolite of Ganoderma lingzhi in liquid fermentation. In order to increase the content of Ganoderma lingzhi EPS, this study added Vernonia amygdala leaf powder to the fermentation medium, and used single factor experiments and orthogonal experiments to optimize fermentation conditions. Fourier transform infrared spectroscopy was used to characterize the structure of Ganoderma lingzhi EPS, then its antioxidant activity was detected. The single factor experiment found that when the optimal addition amount of Vernonia amygdala leaf powder was 4g/L, the content of EPS increased by 167% compared with the control group. The optimum fermentation conditions of the orthogonal experiment were as follows: Fermentation duration was 12 d, initial pH was 5.0, the rotation speed was 120 r/min, and the addition amount of Vernonia amygdala leaf powder was 4 g/L. Under such a condition, the content of EPS could reach 13.05 g/L. The infrared spectra showed that the major absorption peaks of the Ganoderma lingzhi EPS from the addition of Vernonia amygdala leaf powder were similar to those of the EPS from the control group. The antioxidant activity assay showed that the addition of Vernonia amygdala leaf powder had less effect on the scavenging of ABTS+ radicals by EPS, but enhanced their ability to scavenge DPPH radicals, OH radicals, and ferric ion reduction. The results showed that Vernonia amygdala leaf could effectively increase the content of EPS in Ganoderma lingzhi, which provided a new idea for the efficient production of polysaccharides in Ganoderma lingzhi.
-
灵芝(Ganoderma lingzhi)曾用名亮盖灵芝(Ganoderma lucidum),是真菌界担子菌门多孔菌目灵芝科灵芝属(Ganoderma)的大型药用真菌[1]。灵芝主要活性成分为灵芝多糖和灵芝三萜。灵芝多糖具有降血糖、降血脂、抗癌、免疫调节作用以及抗氧化等生理活性[2−3]。目前,灵芝多糖主要来源于孢子粉、子实体、菌丝体和灵芝发酵液等材料[4]。灵芝液体发酵产生的生物活性物质种类与灵芝子实体基本一致[5],且灵芝液体深层发酵具有周期短、成本低、易操作及易工业化生产等优点[6−7],利于灵芝多糖高效生产,逐渐受到人们关注,具有极大的发展潜力。
添加外源物提高灵芝多糖产量已有不少研究,中药材、金属离子、油脂类物质、生长因子等外源物[8−11]可通过诱导、干预和调控灵芝发酵过程中胞外多糖的生物合成及其活性,从而促进灵芝多糖增产。扁桃斑鸠菊在非洲被视为药用植物,其叶子主要活性成分有黄酮类、萜类、生物碱、皂苷等,具有降血脂、抗肿瘤、抗氧化及抗炎等作用[12],扁桃斑鸠菊叶在液态发酵中通常能作为一种添加剂有效提高多糖产量,但是扁桃斑鸠菊叶促进灵芝多糖生产研究尚未见报道。目前,已有报道通过粗毛纤孔菌-扁桃斑鸠菊叶双向液体发酵[13],能有效提高粗毛纤孔菌胞外多糖的含量;候若琳等[14]研究扁桃斑鸠菊叶对蛹虫草胞外多糖的影响,发现扁桃斑鸠菊叶添加量8 g/L时,蛹虫草胞外多糖含量达5.24 g/L,比对照提高205.2%。因此扁桃斑鸠菊叶是食药用菌液态发酵的良好添加剂。
本研究以提高灵芝液体发酵胞外多糖含量为目标,扁桃斑鸠菊叶作为外源物添加至液体发酵培养基,利用单因素和正交试验优化灵芝液体发酵条件,并对灵芝胞外多糖进行红外光谱和抗氧化活性分析,旨在为胞外多糖的高效生产和潜在应用提供科学依据,以促进提升灵芝液体发酵经济效益。
1. 材料与方法
1.1 材料与仪器
灵芝菌种灵芝102、扁桃斑鸠菊叶 由福建农林大学菌物研究中心提供,菌种于4 ℃冰箱贮存,扁桃斑鸠菊叶为春季新鲜采摘,50 ℃烘干;发酵基础培养基为加富的液体培养基(PDB):葡萄糖20 g,酵母浸粉5 g,蛋白胨5 g,MgSO4·7H2O 0.5 g,ZnSO4·7H2O 0.05 g,KH2PO4 1 g,维生素B1 10 mg [15];葡萄糖 上海源叶生物科技有限公司;无水乙醇、过氧化氢、3,5-二硝基水杨酸、酒石酸钾钠、苯酚 生物工程(上海)有限公司;所有试剂均为国产分析纯。
UV-vis1800紫外-可见吸收光谱仪 岛津实验器材有限公司;ST16R高速冷冻离心机、Nicolet iS 50傅立叶变换红外光谱仪 美国Thermo公司;净化工作台 苏州净化设备有限公司;N-001旋转蒸发仪 上海艾郎仪器有限公司;HWS12电热恒温水浴锅、THZ-98 AB恒温摇床 上海一恒科技仪器有限公司;CP214分析天平 上海奥豪斯仪器有限公司;WP-UP-111-20超纯水设备 四川沃特尔水处理设备有限公司。
1.2 实验方法
1.2.1 液体菌种制备
活化灵芝菌种接入已灭菌的100 mL PDB液体培养基,装于250 mL锥形瓶,置于25 ℃和140 r/min摇床培养5 d,即获得液体菌种。接种之前将菌种用无菌水稀释1倍,备用。
1.2.2 胞外粗多糖获取
向装有90 mL PDB液体培养基的锥形瓶中添加适量扁桃斑鸠菊叶粉末,pH自然,灭菌后冷却至室温,接种10 mL液体菌种,置于25 ℃在一定转速的摇床上避光培养一定时间。锥形瓶发酵培养结束,将其液体培养基用双层滤纸抽滤收集滤液,得到含胞外粗多糖的滤液。
1.2.3 胞外多糖测定
1.2.3.1 粗多糖含量测定
取滤液10 mL加入3倍体积无水乙醇,4 ℃过夜,10000 r/min离心15 min,去除上清液,沉淀用10 mL蒸馏水溶解,Sevag法脱蛋白3次,置摇床快速振荡30 min,8000 r/min 离心1 min,取上清液1 mL定容至10 mL,即粗多糖溶液。采用苯酚-硫酸法[16]测定粗多糖含量,即吸取0.1 mL的粗多糖溶液,蒸馏水补充至1 mL,加入现配的5%苯酚溶液1 mL,同时快速并垂直加入5 mL硫酸。摇匀使之充分混合,静置10 min后30 ℃水浴20 min,冷却,490 nm下测定吸光值。每组3个重复。
1.2.3.2 还原糖含量测定
DNS法[17]测定粗多糖溶液中还原糖含量,取粗多糖溶液2.5 mL,加水2.5 mL、DNS 1.5 mL混合均匀,沸水浴5 min,取水浴后的混合液1 mL,蒸馏水补充至20 mL,540 nm下测定吸光值。每组3个重复。
1.2.3.3 胞外多糖含量
参考陈才法等[18]方法计算灵芝胞外多糖含量,即胞外多糖含量=粗多糖含量−还原糖含量。
1.2.4 单因素实验
1.2.4.1 发酵时间
向装有90 mL PDB液体培养基的锥形瓶中添加0.2 g扁桃斑鸠菊叶粉末,pH自然,灭菌后冷却至室温,接种10 mL液体菌种,25 ℃、140 r/min摇床避光培养4、6、8、10、12、14 d,收集滤液,设置3个重复,测定胞外多糖含量。
1.2.4.2 起始pH
固定扁桃斑鸠菊叶粉末添加0.2 g,转速140 r/min,发酵时间6 d,起始pH梯度4.5、5、5.5、6、6.5、7,胞外多糖制备同1.2.4.1。设置3个重复,测定胞外多糖含量。
1.2.4.3 转速
固定扁桃斑鸠菊叶粉末添加0.2 g,发酵时间6 d,pH自然,转速梯度100、120、140、160、180、200 r/min,胞外多糖制备同1.2.4.1。设置3个重复,测定胞外多糖含量。
1.2.4.4 扁桃斑鸠菊叶粉末添加量
固定转速140 r/min,发酵时间6 d,pH自然,扁桃斑鸠菊叶粉末添加0、2、4、6、8、10 g/L,胞外多糖制备同1.2.4.1。设置3个重复,测定胞外多糖含量。
1.2.5 正交优化试验
在单因素实验结果基础上,对发酵时间、起始pH、 转速和扁桃斑鸠菊叶粉末添加量进行4 因素3水平的正交试验,每个试验组重复3次,以胞外多糖含量为考察指标。正交试验设计表见表1。
表 1 胞外多糖发酵条件正交试验因素及水平Table 1. Factors and levels of orthogonal experiment of the fermentation condition of EPS水平 A扁桃斑鸠菊叶粉末
添加量(g/L)B发酵时间
(d)C起始pH D转速
(r/min)1 2 10 4.5 120 2 4 12 5.0 140 3 6 14 5.5 160 1.2.6 红外光谱分析
已去除蛋白质的粗多糖溶液用截留量8000的透析膜透析48 h,除去多余的还原糖等小分子化学物质,冷冻真空干燥,得到灵芝胞外多糖冻干粉。胞外多糖冻干粉10 mg与300 mg溴化钾经高温研磨处理后混合均匀制成压片,红外光谱仪进行检测,扫描范围设定为500~4000 cm−1。
1.2.7 抗氧化活性分析
1.2.7.1 ABTS+·清除率测定
参照Audu等[19]的方法。取不同浓度(0.2、0.4、0.6、0.8、1.0、2.0 g/L)胞外多糖冻干粉配制的样品溶液100 μL和ABTS工作液1.5 mL,加入试管混匀,黑暗静置反应1 h,于波长734 nm处测定吸光值AX;以蒸馏水替代样品溶液测定吸光值A0;以蒸馏水替代ABTS测定吸光值AX0。ABTS+·清除率(%)={1−[(AX−AX0)/A0]}×100,重复3次,取平均值。
1.2.7.2 ·OH清除率测定
采用水杨酸-硫酸亚铁法测定,参照Ketharin等[20]的方法。试管中依次加入不同浓度(0.1、0.2、0.8、1.6、4.0 g/L)胞外多糖冻干粉配制的样品溶液、9 mmol/L的水杨酸-乙醇溶液、9 mmol/L的FeSO4溶液和8.8 mmol/L H2O2溶液各1 mL,最后加蒸馏水至15 mL,摇匀,置37 ℃水浴反应15 min,波长510 nm处测吸光度值AX;以蒸馏水替代样品溶液测定吸光度A0;以蒸馏水替代H2O2测定吸光度AX0。·OH清除率(%)={1−[(AX−AX0)/A0]}×100,重复3次,取平均值。
1.2.7.3 DPPH·清除率测定
参照李欣欣等[21]的方法。取不同浓度(0.4、0.8、1.2、1.6、2.0 g/L)胞外多糖冻干粉配制的样品溶液2 mL和2×10−4 mol/L的DPPH无水乙醇溶液2 mL,加入试管中摇匀,室温下密闭静置30 min,于波长517 nm处测得吸光度AX;以蒸馏水替代样品溶液测定吸光值A0;以蒸馏水替代DPPH测定吸光值AX0。DPPH·清除率(%)={1−[(AX−AX0)/A0]}×100,重复3次,取平均值。
1.2.7.4 Fe3+还原测定
参照Alara等[22]的方法。取不同浓度(0.2、0.4、0.6、1.0、1.2、2.0 g/L)胞外多糖冻干粉配制的样品溶液0.3 mL和FRAP溶液2.7 mL,加入试管混匀,静置反应10 min,波长593 nm处测定吸光值AX;以无水乙醇替代样品溶液测定吸光值A0;以蒸馏水替代FRAP测定吸光值AX0。Fe3+还原值=(AX−AX0)/A0,重复3次,取平均值。
1.3 数据处理
利用DPS软件统计实验数据并绘制直观柱状图,以P<0.05表示组间数据的显著性差异有统计学意义。采用SPSS 13.0软件对正交实验数据进行分析处理,计量数据用平均值(¯x)表示。
2. 结果与分析
2.1 单因素实验结果
2.1.1 发酵时间对胞外多糖含量的影响
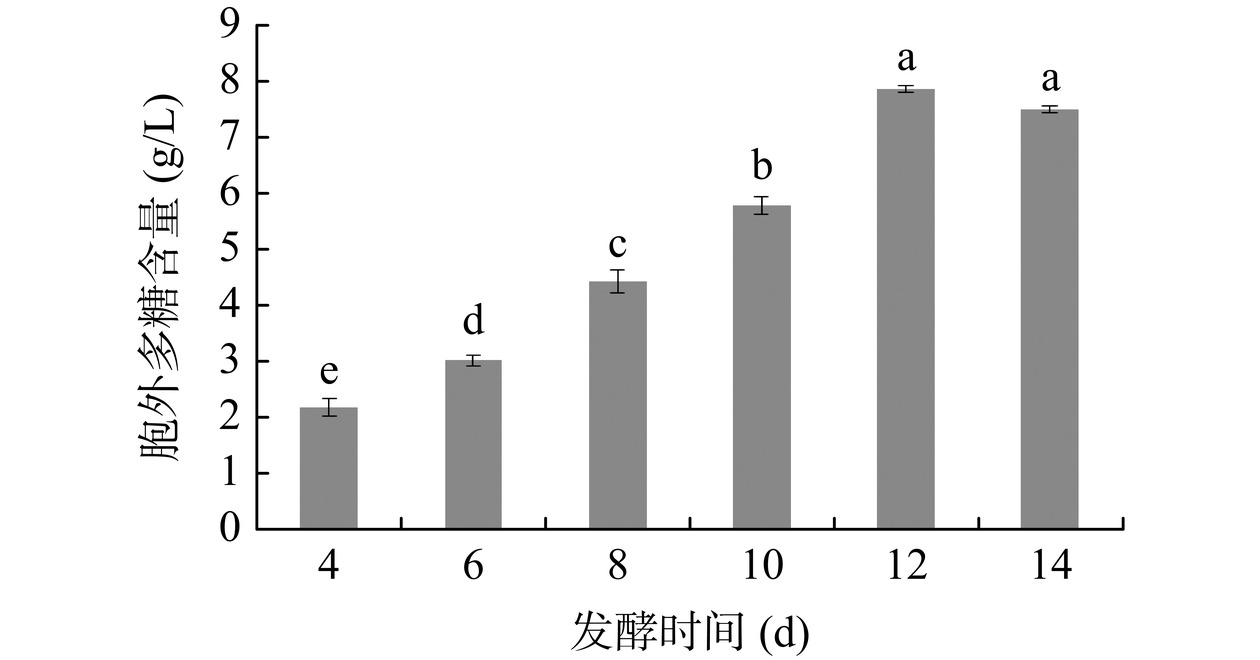
发酵时间对灵芝胞外多糖含量的影响见图1。随发酵时间延长,胞外多糖含量逐渐增加,12 d时达到最大含量,进一步延长发酵时间,胞外多糖含量有一定程度下降。Chien等[23]研究了发酵时间对灵芝液体发酵的影响,也发现灵芝代谢产物随发酵时间增加而先增加后减少,时间过长不利于代谢物的积累。探究其机理,可能是发酵后期菌丝体会对灵芝多糖进行利用,培养基营养物质逐步消耗导致不能满足灵芝菌丝生长需求[24],只能利用发酵液中自身的代谢产物。因此,后续研究选择灵芝胞外多糖最佳的发酵时间12 d。
2.1.2 起始pH对胞外多糖含量的影响
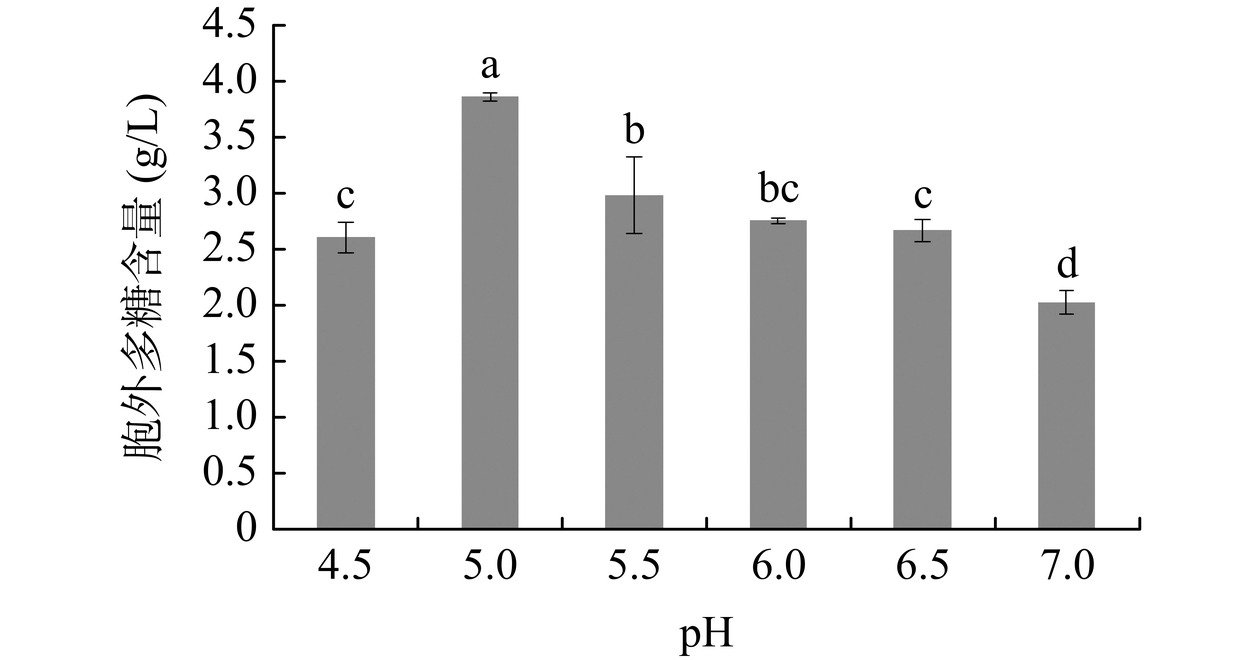
培养基不同起始pH对灵芝胞外多糖含量的影响见图2。起始pH为5时,胞外多糖的含量达到最大,之后随起始pH不断增大,胞外多糖含量逐渐下降,即偏酸性条件有利于多糖合成。与乔双逵等[25]结果一致,即随着起始pH的升高,胞外多糖含量先增加后减少,发酵液过酸或过碱均会导致胞外多糖含量显著减少,可能是过酸或过碱条件抑制胞外多糖合成相关酶的活性[26]。因此,后续研究选择胞外多糖合成的最适起始pH为5。
2.1.3 转速对胞外多糖含量的影响
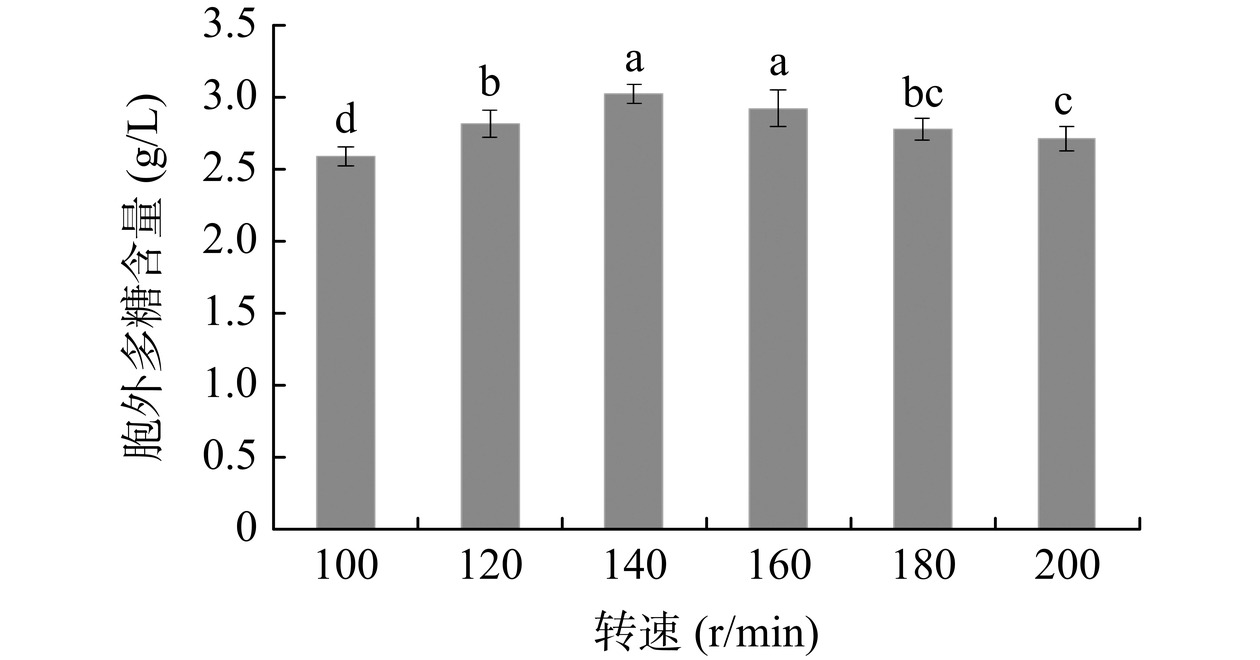
摇床不同转速对灵芝胞外多糖含量的影响见图3。随转速增加,胞外多糖含量相应提高,当转速为140 r/min时达到最大值,继续增大转速,胞外多糖的含量下降。其原因可能是,转速偏低,发酵液中溶氧量不能满足灵芝菌丝生长和新陈代谢,从而使胞外多糖减少。提高转速虽然可以提高溶氧水平,但是周小苹等[27]研究表明溶氧过高不利于灵芝菌丝生长和胞外多糖的合成,可能与过高转速会对菌丝细胞产生损伤有关。因此,后续研究选择摇床最适宜转速为140 r/min。
2.1.4 扁桃斑鸠菊叶粉末添加量对胞外多糖含量的影响
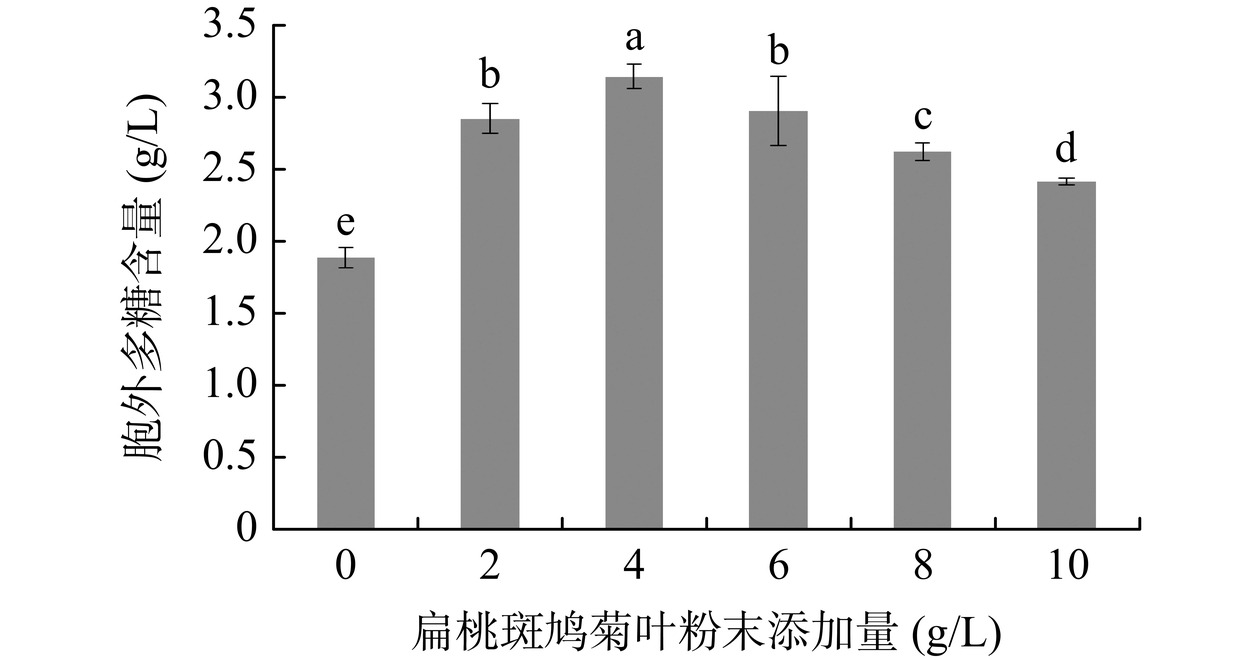
扁桃斑鸠菊叶粉末添加量对灵芝胞外多糖含量的影响见图4。当添加量为4 g/L时,胞外多糖的含量最高,达3.15 g/L,是未添加扁桃斑鸠菊叶粉末的167%。实验结果表明添加扁桃斑鸠菊叶粉末能显著提高灵芝胞外多糖的含量(P<0.05),但添加量过高时,含量反而下降,辛燕花等[28]认为中药材添加量过高会抑制灵芝菌丝体生长,可能是扁桃斑鸠菊叶粉末某些成分达到一定量后对灵芝菌丝生长有抑制作用,从而影响胞外多糖合成。因此,后续研究选择扁桃斑鸠菊叶粉末最适宜的添加量为4 g/L。
为确定扁桃斑鸠菊叶粉末本身含有的多糖是否对胞外多糖含量测定产生影响,采用苯酚硫酸法对扁桃斑鸠菊叶粉末的多糖含量进行了测定,结果显示,扁桃斑鸠菊叶粉末自身多糖含量极低。且实验中添加的扁桃斑鸠菊叶粉末量较少(4 g/L),25 ℃发酵条件下扁桃斑鸠菊叶粉末溶出的多糖少[9],因此扁桃斑鸠菊叶粉末本身对灵芝胞外多糖含量测定的影响可忽略不计。
2.2 液体发酵正交试验优化结果
根据单因素实验结果,对扁桃斑鸠菊叶粉末添加量、发酵时间、起始pH和转速进行四因素三水平的正交试验(表2)。
表 2 正交试验结果直观分析Table 2. Intuitional analysis of orthogonal test results试验号 A B C D EPS含量(g/L) 1 1 1 1 1 9.23 2 1 2 3 3 11.43 3 1 3 2 2 6.92 4 2 1 3 2 10.00 5 2 2 2 1 13.05 6 2 3 1 3 6.82 7 3 1 2 3 9.72 8 3 2 1 2 12.21 9 3 3 3 1 7.53 K1 27.58 28.95 28.26 30.11 K2 30.17 36.98 29.99 29.12 K3 29.46 21.27 28.95 27.97 k1 9.19 9.65 9.42 10.04 k2 10.06 12.33 10.00 9.71 k3 9.82 7.09 9.65 9.32 R 0.87 5.24 0.58 0.71 最佳水平 2 2 2 1 正交试验结果见表2,扁桃斑鸠菊叶粉末添加量的k2最大,发酵时间k2最大,pH k2最大,转速中k1最大,因此,灵芝液体发酵胞外多糖的最佳条件为:扁桃斑鸠菊叶粉末添加量为4 g/L、发酵时间为12 d、起始pH5.0、转速为120 r/min。在最佳条件下,胞外多糖含量达到 13.05 g/L。
表2极差分析表明,4种影响因素中发酵时间极差值最高,为5.24,其次是扁桃斑鸠菊叶粉末添加量,表明添加扁桃斑鸠菊叶粉末的灵芝液体发酵产胞外多糖的发酵工艺,主要影响因子是发酵时间,其次是扁桃斑鸠菊叶粉末添加量。
根据方差分析可知(表3),发酵时间F值最大,为53.862,其次是扁桃斑鸠菊叶粉末添加量,F值为1.566,pH的F值最小,为0.665,4个因素对多糖合成均有显著影响(P<0.05)。因此,4种因素的显著性差异从大到小依次为:发酵时间>扁桃斑鸠菊叶粉末添加量>转速>起始pH 。
表 3 正交试验结果的方差分析Table 3. Variance analysis of orthogonal test results因素 平方和 自由度 均方 F值 显著性 A 1.197 2 0.599 1.566 <0.01 B 41.176 2 20.588 53.862 <0.01 C 0.509 2 0.254 0.665 <0.05 D 0.765 2 0.382 1.122 <0.01 误差 0.612 18 0.034 总和 44.259 26 2.3 红外光谱分析
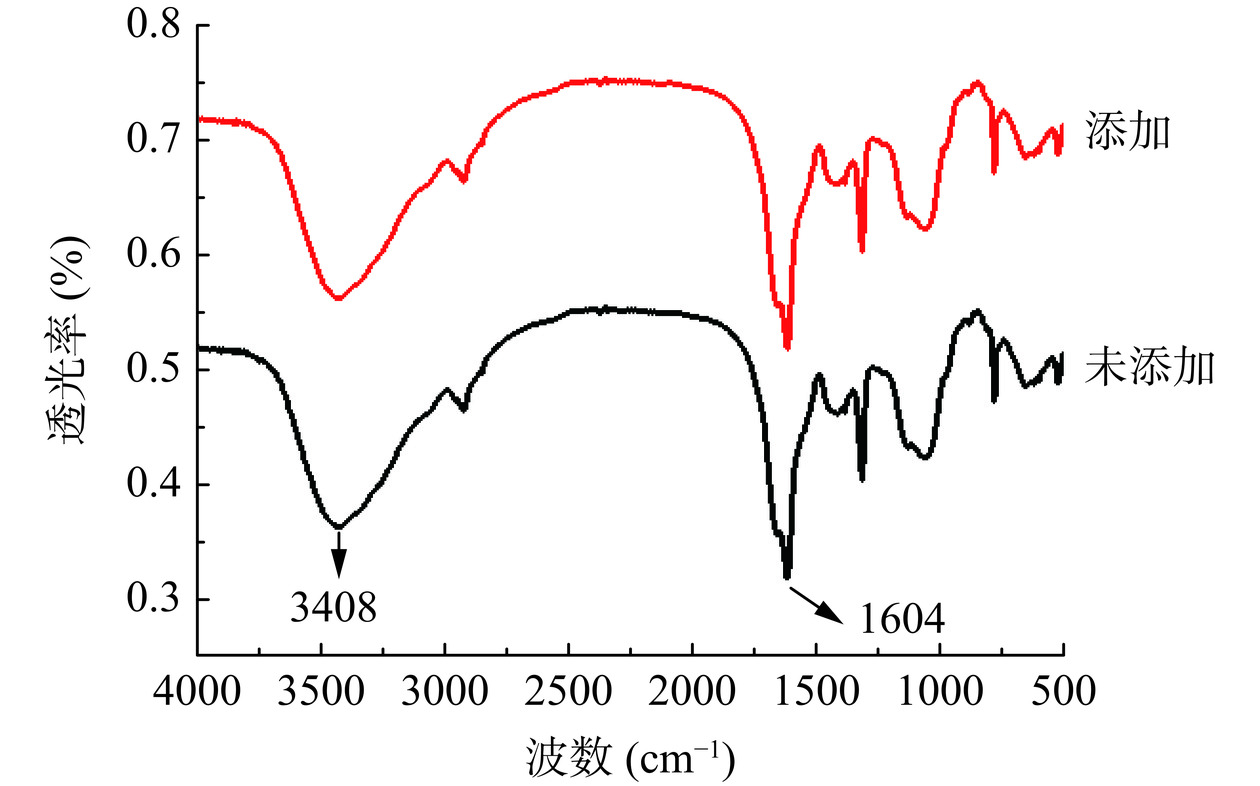
红外光谱分析见图5,添加和未添加扁桃斑鸠菊叶粉末的灵芝胞外多糖的红外光谱在3250~3500 cm−1和1500~1750 cm−1均有多糖的特征吸收峰,3400 cm−1附近吸收峰是羟基O-H的伸缩振动峰[29],1600 cm−1附近吸收峰是羧基COO-的C=O非对称伸缩振动[30],说明多糖组分存在糖醛酸。两者红外光谱图的峰形和峰位置相似,说明其多糖类型相似。
2.4 抗氧化活性分析
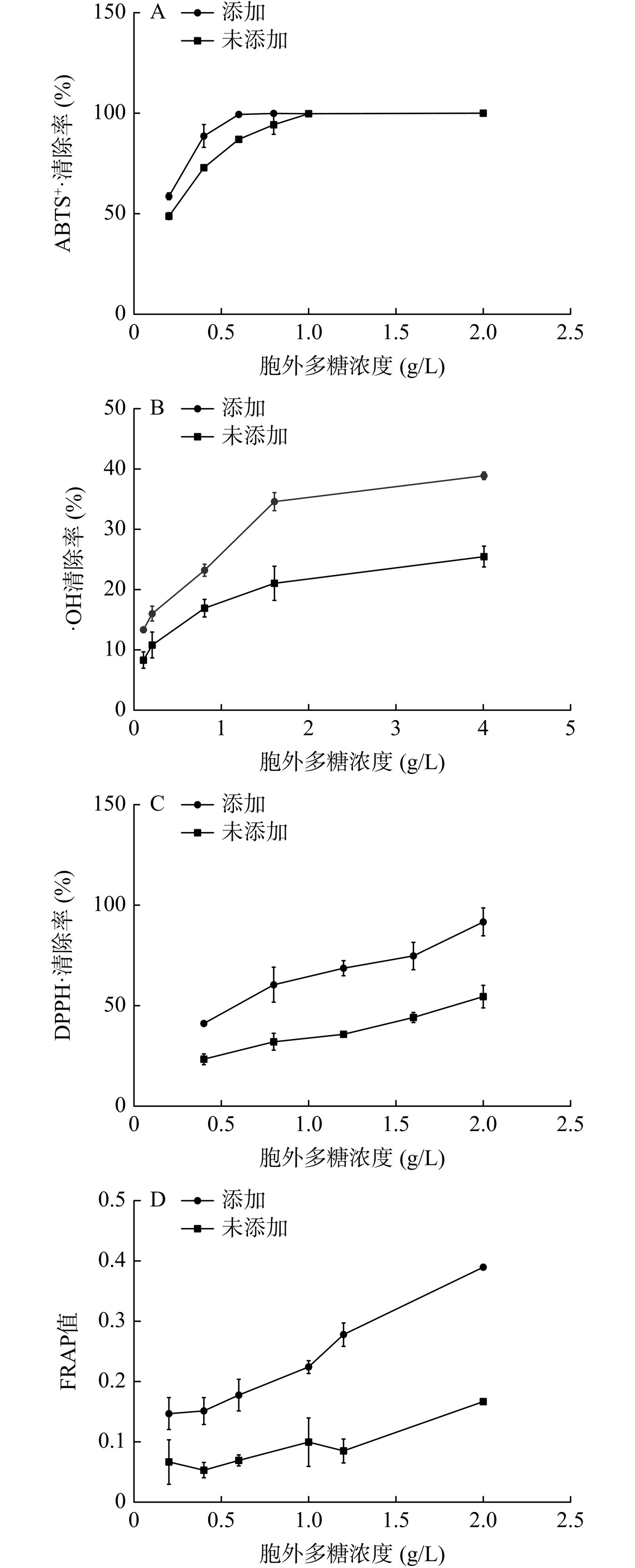
添加扁桃斑鸠菊叶粉末对灵芝胞外多糖抗氧化活性的影响如图6所示。图6A显示在0.2~1.0 g/L范围内,相同胞外多糖浓度下,添加扁桃斑鸠菊叶粉末对ABTS+·的清除能力更强;当胞外多糖浓度高于1.0 g/L,两者清除率相同,添加扁桃斑鸠菊叶粉末清除ABTS+·的IC50为0.15 g/L,而未添加的IC50为0.21 g/L。图6B、C和D显示随胞外多糖浓度增加,对·OH、DPPH·的清除能力和Fe3+还原能力逐渐增强,相同浓度下添加扁桃斑鸠菊叶粉末的多糖自由基清除能力和还原能力更强。在 0.1~4.0 g/L 内,对 ·OH的清除率由13.35%提升至38.9%;在0.4~2.0 g/L内,对DPPH·的清除率由41.14%提升至91.72%;在0.2~2.0 g/L内,对Fe3+还原能力由0.15提升至0.39。通过SPSS软件probit回归计算得出,添加扁桃斑鸠菊叶粉末的胞外多糖清除·OH和DPPH·的IC50分别为10.83、0.52 g/L;而未添加的IC50分别为89.01、1.84 g/L。IC50值越小,对自由基清除作用越强,其抗氧化活性越强,表明添加扁桃斑鸠菊叶粉末后胞外多糖的抗氧化活性有明显的优势。
3. 结论
本文系统研究了扁桃斑鸠菊叶对灵芝胞外多糖合成的影响。通过单因素实验和正交实验优化得到的最佳发酵条件为:发酵时间12 d,起始pH5.0,转速120 r/min,扁桃斑鸠菊叶粉末的添加量为4 g/L;在此条件下,胞外多糖含量达到 13.05 g/L。红外光谱分析表明添加和未添加扁桃斑鸠菊叶粉末的灵芝胞外多糖类型相似,均含O-H和C=O振动吸收峰,但对灵芝胞外多糖抗氧化活性有较大影响,添加扁桃斑鸠菊叶粉末的胞外多糖自由基清除能力和还原能力更强。关于扁桃斑鸠菊叶如何促进灵芝合成胞外多糖以及影响多糖生物活性机制,需要进一步的实验探究。
-
表 1 胞外多糖发酵条件正交试验因素及水平
Table 1 Factors and levels of orthogonal experiment of the fermentation condition of EPS
水平 A扁桃斑鸠菊叶粉末
添加量(g/L)B发酵时间
(d)C起始pH D转速
(r/min)1 2 10 4.5 120 2 4 12 5.0 140 3 6 14 5.5 160 表 2 正交试验结果直观分析
Table 2 Intuitional analysis of orthogonal test results
试验号 A B C D EPS含量(g/L) 1 1 1 1 1 9.23 2 1 2 3 3 11.43 3 1 3 2 2 6.92 4 2 1 3 2 10.00 5 2 2 2 1 13.05 6 2 3 1 3 6.82 7 3 1 2 3 9.72 8 3 2 1 2 12.21 9 3 3 3 1 7.53 K1 27.58 28.95 28.26 30.11 K2 30.17 36.98 29.99 29.12 K3 29.46 21.27 28.95 27.97 k1 9.19 9.65 9.42 10.04 k2 10.06 12.33 10.00 9.71 k3 9.82 7.09 9.65 9.32 R 0.87 5.24 0.58 0.71 最佳水平 2 2 2 1 表 3 正交试验结果的方差分析
Table 3 Variance analysis of orthogonal test results
因素 平方和 自由度 均方 F值 显著性 A 1.197 2 0.599 1.566 <0.01 B 41.176 2 20.588 53.862 <0.01 C 0.509 2 0.254 0.665 <0.05 D 0.765 2 0.382 1.122 <0.01 误差 0.612 18 0.034 总和 44.259 26 -
[1] 崔宝凯, 吴声华. 普遍栽培灵芝种类的拉丁学名[J]. 菌物学报,2020,39(1):7−12. [CUI B K, WU S H. The scientific name of the widely cultivated Ganoderma species[J]. Mycosystema,2020,39(1):7−12.] CUI B K, WU S H. The scientific name of the widely cultivated Ganoderma species[J]. Mycosystema, 2020, 39(1): 7−12.
[2] 孟歌, 崔宝凯, 李春道, 等. 药用真菌灵芝液体培养过程中的抗氧化活性研究[J]. 菌物学报,2018,37(4):486−501. [MENG G, CUI B K, LI C D, et al. Antioxidant activities of medicinal fungus Ganoderma lingzhi in the process of liquid cultivation[J]. Mycosystema,2018,37(4):486−501.] MENG G, CUI B K, LI C D, et al. Antioxidant activities of medicinal fungus Ganoderma lingzhi in the process of liquid cultivation[J]. Mycosystema, 2018, 37(4): 486−501.
[3] ZENG X, LI P, CHEN X, et al. Effects of deproteinization methods on primary structure and antioxidant activity of Ganoderma lucidum polysaccharides[J]. International Journal of Biological Macromolecules,2019,126:867−876.
[4] 郭嘉, 冯杰, 谭贻, 等. 灵芝液态发酵胞内外多糖的研究进展[J]. 微生物学通报,2022,49(10):4337−4356. [GUO J, FENG J, TAN Y, et al. Liquid fermentation of Ganoderma lingzhi for intracellular and extracellular polysaccharides review[J]. Microbiology China,2022,49(10):4337−4356.] GUO J, FENG J, TAN Y, et al. Liquid fermentation of Ganoderma lingzhi for intracellular and extracellular polysaccharides review[J]. Microbiology China, 2022, 49(10): 4337−4356.
[5] 陆正清. 灵芝液体深层发酵技术[J]. 江苏食品与发酵,2007(3):38−40. [LU Z Q. Liquid deep fermentation technology of Ganoderma lucidum[J]. Jiangsu Food & Fermentation,2007(3):38−40.] LU Z Q. Liquid deep fermentation technology of Ganoderma lucidum[J]. Jiangsu Food & Fermentation, 2007(3): 38−40.
[6] 解修超, 贾少杰, 彭浩, 等. 灵芝多糖液体发酵调控及药理作用研究进展[J]. 陕西理工大学学报:自然科学版,2018,34(6):65−70. [XIE X C, JIA S J, PENG H, et al. Research progress on regulation of liquid fermentation and pharmacological action of Ganoderma lucidum polysaccharide[J]. Journal of Shaanxi University of Technology:Natural Science Edition,2018,34(6):65−70.] XIE X C, JIA S J, PENG H, et al. Research progress on regulation of liquid fermentation and pharmacological action of Ganoderma lucidum polysaccharide[J]. Journal of Shaanxi University of Technology: Natural Science Edition, 2018, 34(6): 65−70.
[7] 魏滔, 张长生, 陈琼华, 等. 灵芝真菌液体发酵及其产物应用的研究进展[J]. 微生物学通报2022, 49(1):336−351. [WEI T, ZHANG C S, CHEN Q H, et al. Liquid fermentation of Ganoderma and application of its products[J]. Microbiology China, 2022, 49(1):336−351.] WEI T, ZHANG C S, CHEN Q H, et al. Liquid fermentation of Ganoderma and application of its products[J]. Microbiology China, 2022, 49(1): 336−351.
[8] 朱强, 夏艳秋, 汪志君, 等. 4种中药对灵芝生长与发酵的影响[J]. 中国酿造,2010,29(7):163−165. [ZHU Q, XIA Y Q, WANG Z J, et al. Effects of four herbs on growth and fermentation of Ganoderma lucidum[J]. China Brewing,2010,29(7):163−165.] ZHU Q, XIA Y Q, WANG Z J, et al. Effects of four herbs on growth and fermentation of Ganoderma lucidum[J]. China Brewing, 2010, 29(7): 163−165.
[9] 叶盛权, 吴晖, 余以刚, 等. 不同金属离子对灵芝多糖液态发酵的影响[J]. 食品研究与开发,2011,32(1):106−108. [YE S Q, WU H, YU Y G, et al. Research for different metal ion in Ganoderma lucidum polysaccharides fermentation[J]. Food Research and Development,2011,32(1):106−108.] YE S Q, WU H, YU Y G, et al. Research for different metal ion in Ganoderma lucidum polysaccharides fermentation[J]. Food Research and Development, 2011, 32(1): 106−108.
[10] YANG H L, MIN W H, BI P Y, et al. Stimulatory effects of Coix lacryma-jobi oil on the mycelial growth and metabolites biosynthesis by the submerged culture of Ganoderma lucidum[J]. Biochemical Engineering Journal,2013,76:77−82. doi: 10.1016/j.bej.2013.04.012
[11] 曾晓希, 周洪波, 符波, 等. 灵芝多糖液体发酵条件的研究[J]. 现代生物医学进展,2007,7(6):830−832. [ZENG X X, ZHOU H B, FU B, et al. Study on fermentation conditions of Ganoderma lucidum[J]. Progress in Modern Biomedicine,2007,7(6):830−832.] ZENG X X, ZHOU H B, FU B, et al. Study on fermentation conditions of Ganoderma lucidum[J]. Progress in Modern Biomedicine, 2007, 7(6): 830−832.
[12] 郑文豪, 王珍珍, 李江, 等. 扁桃斑鸠菊叶酵素发酵过程中理化性质分析、抗氧化能力评价及对氧化应激细胞防护作用研究[J]. 食品工业科技,2021,42(18):9−17. [ZHENG W H, WANG Z Z, LI J, et al. Physicochemical properties, antioxidant activity and protective effect on oxidative stress cells of Vernonia amygdalina Delile leaf Jiaosu during fermentation[J]. Science and Technology of Food Industry,2021,42(18):9−17.] ZHENG W H, WANG Z Z, LI J, et al. Physicochemical properties, antioxidant activity and protective effect on oxidative stress cells of Vernonia amygdalina Delile leaf Jiaosu during fermentation[J]. Science and Technology of Food Industry, 2021, 42(18): 9−17.
[13] 刘鑫, 李琳, 陈磊, 等. 利用扁桃斑鸠菊叶发酵高产粗毛纤孔菌胞外多糖的条件优化及其抗氧化活性研究[J]. 菌物学报,2019,38(3):403−413. [LIU X, LI L, CHEN L, et al. Optimization on fermentation conditions for high production of Inonotus hispidus exopolysaccharides using Vernonia amygdalina dried leaf powder as additive and analyses on antioxidant activities of the polysaccharide product[J]. Mycosystema,2019,38(3):403−413.] LIU X, LI L, CHEN L, et al. Optimization on fermentation conditions for high production of Inonotus hispidus exopolysaccharides using Vernonia amygdalina dried leaf powder as additive and analyses on antioxidant activities of the polysaccharide product[J]. Mycosystema, 2019, 38(3): 403−413.
[14] 侯若琳, 李琳, 项凯凯, 等. 利用扁桃斑鸠菊叶发酵生产蛹虫草胞外多糖的条件及其抗氧化活性[J]. 生物工程学报,2019,35(4):667−676. [HOU R L, LI L, XIANG K K, et al. Production of antioxidative exopolysaccharides of Cordyceps militaris with Vernonia amygdalina leaves in substrate[J]. Mycosystema,2019,35(4):667−676.] HOU R L, LI L, XIANG K K, et al. Production of antioxidative exopolysaccharides of Cordyceps militaris with Vernonia amygdalina leaves in substrate[J]. Mycosystema, 2019, 35(4): 667−676.
[15] 孟歌. 灵芝产胞外多糖的液体培养基优化及其结构初步解析和抗氧化活性研究[D]. 北京:北京林业大学, 2018. [MENG G. Optimization of liquid medium for exopolysaccharides produced by Ganoderma lucidum and preliminary analysis of its structure and antioxidant activity[D]. Beijing:Beijing Forestry University, 2018.] MENG G. Optimization of liquid medium for exopolysaccharides produced by Ganoderma lucidum and preliminary analysis of its structure and antioxidant activity[D]. Beijing: Beijing Forestry University, 2018.
[16] DUBOIS M, GILLES K A, HAMILTOM J K, et al. Colorimetric method for determination of sugars and related substances[J]. Analytical Chemistry,1956,28(3):350−356. doi: 10.1021/ac60111a017
[17] 张腾霄, 王斌, 张希, 等. 枯草芽孢杆菌对香菇发酵液糖类成分降解规律研究[J]. 食品工业,2017,38(1):184−187. [ZHANG T X, WANG B, ZHANG X, et al. Study on the degradation of sugars in Lentinus edodes fermentation broth by Bacillus subtilis[J]. The Food Industry,2017,38(1):184−187.] ZHANG T X, WANG B, ZHANG X, et al. Study on the degradation of sugars in Lentinus edodes fermentation broth by Bacillus subtilis[J]. The Food Industry, 2017, 38(1): 184−187.
[18] 陈才法, 项小燕, 顾琪, 等. 桦褐孔菌胞外多糖合成的深层发酵条件研究[J]. 中草药,2007,38(3):358−361. [CHEN C F, XIANG X Y, GU Q, et al. Submerged fermentation conditions for synthesis of extracellular polysaccharide by Inonotus obliquus[J]. Chinese Traditional and Herbal Drugs,2007,38(3):358−361.] doi: 10.3321/j.issn:0253-2670.2007.03.016 CHEN C F, XIANG X Y, GU Q, et al. Submerged fermentation conditions for synthesis of extracellular polysaccharide by Inonotus obliquus[J]. Chinese Traditional and Herbal Drugs, 2007, 38(3): 358−361. doi: 10.3321/j.issn:0253-2670.2007.03.016
[19] AUDU S A, TAIWO A E, OJUOLAPE A R. A study review of documented phytochemistry of Vernonia amygdalina (family asteraceae) as the basis for pharmacologic activity of plant extract[J]. Natl Sci Res,2012,2(7):1−8.
[20] KETHARIN L, NAGARAJA S, THEVANAYAGY A. Screening of Vernonia amygdalina leaf extracts for antioxidant and antimicrobial activity[J]. Materials Today:Proceedings,2019,16(4):1809−1818.
[21] 李欣欣, 李文香. 桦褐孔菌多糖的分离纯化及其抗氧化活性测定[J]. 食品工业科技,2021,42(11):192−197. [LI X X, LI W X. Isolation, purification and antioxidant activity of Inonotus obliquus polysaccharide[J]. Science and Technology of Food Industry,2021,42(11):192−197.] LI X X, LI W X. Isolation, purification and antioxidant activity of Inonotus obliquus polysaccharide[J]. Science and Technology of Food Industry, 2021, 42(11): 192−197.
[22] ALARA O R, ABDURAHMAN N H, OLALERRE O A. Mathematical modelling and morphological properties of thin layer oven drying of Vernonia amygdalina leaves[J]. Journal of the Saudi Society of Agricultural Sciences,2019,18(3):309−315. doi: 10.1016/j.jssas.2017.09.003
[23] CHIEN L Y, HO C T, CHIANG B H, et al. Effect of fermentation time on antioxidative activities of Ganoderma lucidum broth using leguminous plants as part of the liquid fermentation medium[J]. Food Chemistry,2011,126(4):1586−1592. doi: 10.1016/j.foodchem.2010.12.024
[24] 张雪洪, 胡洪波, 唐涌濂, 等. 膜生物反应器连续发酵生产胞外灵芝多糖[J]. 高校化学工程学报,2002,16(6):670−674. [ZHANG X H, HU H B, TANG Y L, et al. Continuous fermentation of Ganoderma lucidum exo-polysaccharide in a membrane bioreactor[J]. Journal of Chemical Engineering of Chinese Universities,2002,16(6):670−674.] doi: 10.3321/j.issn:1003-9015.2002.06.014 ZHANG X H, HU H B, TANG Y L, et al. Continuous fermentation of Ganoderma lucidum exo-polysaccharide in a membrane bioreactor[J]. Journal of Chemical Engineering of Chinese Universities, 2002, 16(6): 670−674. doi: 10.3321/j.issn:1003-9015.2002.06.014
[25] 乔双逵, 彭林, 丁重阳, 等. 液体发酵条件对灵芝菌体形态及胞外多糖活性的影响[J]. 食品与生物技术学报,2014,33(10):1070−1076. [QIAO S K, PENG L, DING C Y, et al. Effect of different culture conditions on mycelium morphology and activity of exo-polysaccharides from Ganoderma lucidum in submerged culture[J]. Journal of Food Science and Biotechnology,2014,33(10):1070−1076.] QIAO S K, PENG L, DING C Y, et al. Effect of different culture conditions on mycelium morphology and activity of exo-polysaccharides from Ganoderma lucidum in submerged culture[J]. Journal of Food Science and Biotechnology, 2014, 33(10): 1070−1076.
[26] FANG Q H, ZHONG J J. Effect of initial pH on production of ganoderic acid and polysaccharide by submerged ferm entation of Ganoderma lucidum[J]. Process Biochemistry,2002,37(7):769−774. doi: 10.1016/S0032-9592(01)00278-3
[27] 周小苹, 杨海军. 溶氧对灵芝液体深层培养产胞外多糖的影响[J]. 湖北农业科学,2011,50(18):3804−3807. [ZHOU X P, YANG H J. Effects of dissolved oxygen on exo-cellular polysaccharides produced by deep liquid cultivation of Ganoderma lucidum[J]. Hubei Agricultural Sciences,2011,50(18):3804−3807.] doi: 10.3969/j.issn.0439-8114.2011.18.040 ZHOU X P, YANG H J. Effects of dissolved oxygen on exo-cellular polysaccharides produced by deep liquid cultivation of Ganoderma lucidum[J]. Hubei Agricultural Sciences, 2011, 50(18): 3804−3807. doi: 10.3969/j.issn.0439-8114.2011.18.040
[28] 辛燕花, 张铁丹, 张建华, 等. 灵芝-何首乌双向液体发酵菌质抗氧化活性研究[J]. 食用菌学报,2018,25(3):63−71. [XIN Y H, ZHANG T D, ZHANG J H, et al. Antioxidant capacity of fungal substance from Bi-directional fermentation of Ganoderma lucidum and Polygonum multiflorum[J]. Acta Edulis Fungi,2018,25(3):63−71.] XIN Y H, ZHANG T D, ZHANG J H, et al. Antioxidant capacity of fungal substance from Bi-directional fermentation of Ganoderma lucidum and Polygonum multiflorum[J]. Acta Edulis Fungi, 2018, 25(3): 63−71.
[29] 张瑞平, 任昭辉, 张皓楠, 等. 香加皮多糖的分离纯化、单糖组成及其抗氧化活性研究[J]. 2023, 44(13):71-78. [ZHANG R P, REN Z H, ZHANG H N, et al. Isolation, purification, monosaccharide composition and antioxidant activity of polysaccharides from Cortex Periplocae[J]. Science and Technology of Food Industry, 2023, 44(13):71-78.] ZHANG R P, REN Z H, ZHANG H N, et al. Isolation, purification, monosaccharide composition and antioxidant activity of polysaccharides from Cortex Periplocae[J]. Science and Technology of Food Industry, 2023, 44(13): 71-78.
[30] WANG L, ZHANG B, XIAO J, et al. Physicochemical, functional, and biological properties of water-soluble polysaccharides from Rosa roxburghii Tratt fruit[J]. Food Chemistry,2018,249:127−135. doi: 10.1016/j.foodchem.2018.01.011





 下载:
下载:
 下载:
下载:
